Brain Facts - A Primer on the Brain & Nervous System, SFN, BrainFacts.org
Principles of Neural Science, (5th Ed. pdf)
Neuroscience posters: Neurodegeneration, Neuroinflammation
Oh et al., Scientific Reports, 2022.03
“Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is accompanied by chronic neurological sequelae such as cognitive decline and mood disorder, but the underlying mechanisms have not yet been elucidated. We explored the possibility that the brain-infiltrating SARS-CoV-2 spike protein contributes to the development of neurological symptoms observed in COVID-19 patients in this study. Our behavioral study showed that administration of SARS-CoV-2 spike protein S1 subunit (S1 protein) to mouse hippocampus induced cognitive deficit and anxiety-like behavior in vivo. These neurological symptoms were accompanied by neuronal cell death in the dorsal and ventral hippocampus as well as glial cell activation. Interestingly, the S1 protein did not directly induce hippocampal cell death in vitro. Rather, it exerted neurotoxicity via glial cell activation, partially through interleukin-1β induction. In conclusion, our data suggest a novel pathogenic mechanism for the COVID-19-associated neurological symptoms that involves glia activation and non-cell autonomous hippocampal neuronal death by the brain-infiltrating S1 protein.”
Fig. 2 SARS-CoV-2 S1 protein induces hippocampal neuronal death in CA1 and DG areas. (a-b). Hippocampal neurons in mice injected with S1 protein (n = 5) or vehicle (Control, n = 5) were visualized by cresyl violet staining (purple, scale bars = 200, 50, and 200 µm for left, middle, and right of dorsal area; 500 µm for left and 125 µm for middle and right of ventral area) and immunostaining with NeuN antibody (red, scale bars = 200, 50, and 100 µm for left, middle, and right of dorsal area; 500 µm for left and 125 µm for middle and right of ventral area). Hippocampal slices were prepared 14 days post-injection. (c) NeuN-positive cells in the CA1 and DG regions of the dorsal and ventral hippocampus were manually counted using ImageJ software (Wayne Rasband, National Institutes of Health, USA) in a blind manner. Data are presented as the mean ± s.e.m. Statistical results are for unpaired t-tests. **** p < 0.0001, ** p < 0.01, * p < 0.05.
SARS-CoV-2 is associated with changes in brain structure in UK Biobank
Douaud et al., Nature, 2022.03
“There is strong evidence for brain-related abnormalities in COVID-19. … We identified significant longitudinal effects when comparing the two groups, including: (i) greater reduction in grey matter thickness and tissue-contrast in the orbitofrontal cortex and parahippocampal gyrus, (ii) greater changes in markers of tissue damage in regions functionally-connected to the primary olfactory cortex, and (iii) greater reduction in global brain size. The infected participants also showed on average larger cognitive decline between the two timepoints.
Cognitive results: Using the main model used to compare longitudinal imaging effects between SARS-CoV-2 positive participants and controls (Model 1), we explored differences between the two groups in 10 scores from 6 cognitive tasks. These 10 scores were selected using a data-driven approach based on out-of-sample participants who are the most likely to show cognitive impairment. After FDR correction, we found a significantly greater increase of the time taken to complete Trails A (numeric) and B (alphanumeric) of the Trail Making Test in the SARS-CoV-2 infected group.
Discussion: To our knowledge, this is the first longitudinal imaging study of SARS-CoV-2 where participants were initially scanned before any had been infected. Our longitudinal analyses revealed a significant, deleterious impact associated with SARS-CoV-2. This impact could be seen mainly in the limbic and olfactory cortical system, for instance with a change in diffusion measures — that are proxies for tissue damage — in regions functionally connected with the piriform cortex, olfactory tubercle and anterior olfactory nucleus, as well as a more pronounced reduction of grey matter thickness and contrast in the SARS-CoV-2 infected participants in the left parahippocampal gyrus and lateral orbitofrontal cortex.
Finally, significantly greater cognitive decline, which persisted even after excluding the hospitalised patients, was seen in the SARS-CoV-2 positive group between the two timepoints, and this decline was associated with greater atrophy of crus II, a cognitive lobule of the cerebellum.
The overlapping olfactory- and memory-related functions of the regions shown to alter significantly over time in SARS-CoV-2, including the parahippocampal gyrus/perirhinal cortex, entorhinal cortex and hippocampus in particular, raise the possibility that longer-term consequences of SARS-CoV-2 infection might in time contribute to Alzheimer’s disease or other forms of dementia.”
Fig. 2. Vertex-wise and voxel-wise longitudinal group differences in grey matter thickness and mean diffusivity changes. Top row. Main analysis (Model 1): The thresholded map (|Z|>3) shows that the strongest, localised reduction of grey matter thickness in the 401 SARS-CoV-2 positive participants compared with the 384 controls are bilaterally in the parahippocampal gyrus, anterior cingulate cortex and temporal pole, as well as in the left orbitofrontal cortex, insula and supramarginal gyrus.
Cognitive decline and brainstem hypometabolism in long COVID: A case series
Hugon et al., Brain and Behavior, 2022.03
Results: “We report here 3 cases of brain fog with major hypometabolic areas of the pons revealed by the cerebral FDG PET.
Conclusion: The dysfunction of the locus coeruleus in these patients could partly explain the cognitive disorders observed. Further studies involving larger cohorts of patients suffering from cognitive dysfunction will be needed to determine if the brainstem is frequently affected in these patients.
In conclusion, these findings could suggest that cognitive deficits observed in these COVID patients could partly originate from the dysfunction of the locus coeruleus. Our results also emphasize the need to explore COVID patients complaining of cognitive deficits with cerebral FDG PET even in non-hospitalized subjects affected by mild Sars-Cov-2 infections.”
Figure 1 The results of the FDG PET imaging in the three patients. In patient 1, only the pons was mainly hypometabolic. In patient 2, in addition to the pons, the precuneus and the left parietal areas were hypometabolic. In patient 3, the pons and the cingulate cortex were found to be hypometabolic
SARS-CoV-2 invades cognitive centers of the brain and induces Alzheimer's-like neuropathology
Shen et al., bioRxiv, 2022.02.01
“Major cell entry factors of SARS-CoV-2 are present in neurons; however, the neurotropism of SARS-CoV-2 and the phenotypes of infected neurons are still unclear. Acute neurological disorders occur in many patients, and one-third of COVID-19 survivors suffer from brain diseases. Here, we show that SARS-CoV-2 invades the brains of five patients with COVID-19 and Alzheimers, autism, frontotemporal dementia or no underlying condition by infecting neurons and other cells in the cortex. SARS-CoV-2 induces or enhances Alzheimers-like neuropathology with manifestations of beta-amyloid aggregation and plaque formation, tauopathy, neuroinflammation and cell death. SARS-CoV-2 infects mature but not immature neurons derived from inducible pluripotent stem cells from healthy and Alzheimers individuals through its receptor ACE2 and facilitator neuropilin-1. SARS-CoV-2 triggers Alzheimers-like gene programs in healthy neurons and exacerbates Alzheimers neuropathology. A gene signature defined as an Alzheimers infectious etiology is identified through SARS-CoV-2 infection, and silencing the top three downregulated genes in human primary neurons recapitulates the neurodegenerative phenotypes of SARS-CoV-2. Thus, SARS-CoV-2 invades the brain and activates an Alzheimers-like program.”
Figure 3. Spike protein-positive cells undergo programmed cell death.
“Representative images of double SARS-CoV-2 spike protein (SP) (Ni-DAB staining: black signal) and cleaved caspase 3 (cl-casp3; AP: red signal) staining in the cortexes of age-matched non-COVID-19 autism and COVID-19 autism cases (A) and non-COVID-19 Alzheimer’s and the COVID-19 Alzheimer’s case (B). The inset boxes show cl-casp3 and SP double-labeled cells, cl-casp3-positive cells and double-negative cells.
The graphs show the percentages of double SP+ and cl-casp3+ cells among total cleaved caspase 3+ cells in at least 5 random fields. * indicate significant difference (P < 0.05) compared with the non-COVID-19 controls by t test.
Representative images of SP staining with the necroptosis marker phospho-MLKL (C), the ferroptosis marker TfR1 (D), and the senescence marker DPP4 (E) in the cortexes of the non-COVID-19 autism cases, the COVID-19 Alzheimer’s case and the two COVID-19 autism cases. Black arrows point to the double SP+ and cl-casp3+ cells.
The graphs show the percentages of SP+ necroptotic cells, ferroptotic cells and senescent cells counted from at least 5 random fields (n > 5, in 2 COVID-19 ASD cases and 1 COVID-19 AD case). * indicate significant difference (P < 0.05) compared with the non-COVID-19 controls by one-way ANOVA and Tukey test. Scale bars = 20 μm.”
Alzheimer's-like signaling in brains of COVID-19 patients
Reiken et al., Alzheimer’s & Dementia, 2022.02
Introduction: “The mechanisms that lead to cognitive impairment associated with COVID-19 are not well understood.”
Results: “We provide evidence linking SARS-CoV-2 infection to activation of TGF-β signaling and oxidative overload. The neuropathological pathways causing tau hyperphosphorylation typically associated with AD were also shown to be activated in COVID-19 patients. RyR2 in COVID-19 brains demonstrated a “leaky” phenotype, which can promote cognitive and behavioral defects.”
Discussion: “COVID-19 neuropathology includes AD-like features and leaky RyR2 channels could be a therapeutic target for amelioration of some cognitive defects associated with SARS-CoV-2 infection and long COVID.”
Fig. 4. SARS-CoV-2 infection results in leaky ryanodine receptor 2 (RyR2) that may contribute to cardiac, pulmonary, and cognitive dysfunction. SARS-CoV-2 infection targets cells via the angiotensin-converting enzyme 2 (ACE2) receptor, inducing inflammasome stress response/activation of stress signaling pathways. This results in increased transforming growth factor-β (TGF-β) signaling, which activates SMAD3 (pSMAD) and increases NADPH oxidase 2 (NOX2) expression and the amount of NOX2 associated with RyR2. Increased NOX2 activity at RyR2 oxidizes the channel, causing calstabin2 depletion from the channel macromolecular complex, destabilization of the closed state, and ER/SR calcium leak that is known to contribute to cardiac dysfunction,55 arrhythmias,61 pulmonary insufficiency,23, 25 and cognitive and behavioral abnormalities associated with neurodegenreation.24, 26 Decreased calbindin in COVID-19 may render brain more susceptible to tau pathology. Rycal drugs fix the RyR2 channel leak by restoring calstabin2 binding and stabilizing the channel closed state. Fixing leaky RyR2 may improve cardiac, pulmonary, and cognitive function in COVID-19.
Long COVID, neuropsychiatric disorders, psychotropics, present and future
Tang et al., Acta Neuropsychiatrica, 2022.02
Neurological complications
“The neurological complications/consequences in long COVID can be largely grouped into three major categories:
Direct viral invasion of the brain neuronal and vascular structures and its consequences
Abnormal immune and inflammatory reaction such as ‘cytokine storm’ and its long-term consequences
Neurological consequences secondary to viral pulmonary and associated systemic disease including systemic inflammation, sepsis and multi-organ failure, resulting in hypoxic brain damage, encephalopathy and stroke, Guillain–Barré syndrome (GBS), acute haemorrhagic necrotising encephalopathy (ANE) and acute disseminated encephalomyelitis (ADEM)
Long COVID neurodegeneration
Normal CNS neuronal mitochondrial function requires high oxygen levels. SARS-CoV-2 virus can hijack mitochondrial function to cause long-lasting metabolic problems. Coupled with the inflammatory process discussed above, neurodegeneration or exacerbation of pre-existing dementia may begin.
COVID-19 neuroinflammation may lead to decreased neurogenesis, as shown in a reduction in the size of the hippocampus, dentate gyrus and fewer granule neurons and neural progenitor cells. It is already well-established that cognitive dysfunction may persist for many months after patients have apparently recovered from COVID-19. This points to the possibility that there is long-term damage to the neuronal networks initiated by the virus and extended and sustained by chronic neuroinflammation and the disruption of brain metabolic homeostasis.”
First episode psychosis following receipt of first dose of COVID-19 vaccine: A case report
Grover et al., Schizophrenia Research, 2022.01
“Very few reports are available showing COVID-19 vaccine induced psychosis. Studies have shown that SAR-CoV-2 is known to trigger a powerful immune response, which includes the release of large amounts of pro-inflammatory cytokines. It is hypothesized that psychosis may be related to rapid increase in the proinflammatory response and activated autoimmune mechanism (Yesilkaya et al., 2021). The MRI findings of tiny T2/FLAIR hyperintensities in deep white matter in bilateral fronto-parietal lobes in the index case could be indicators of the endothelial and autoimmune activation, as seen in autoimmune encephalitis.
It has also been hypothesized that COVID-19 may increase the risk of psychosis by triggering the cytokine storm. It can be hypothesized that the cytokine storm with elevated serum concentrations of IL-6, IL-8 and IL-2 can affect the levels of monoamine neurotransmitters, i.e., lead to increase reuptake of dopamine, serotonin, and norepinephrine, and influence on the release of neurotransmitters. Cytokines may also result in increased kynurenic acid with resultant NMDA receptor hypofunction, increased pyramidal firing, increased inhibitory activity of the nucleus accumbens, and decreased inhibitory tone over the ventral tegmental area dopaminergic neurons and increased production of dopamine. The increase in the dopamine may be responsible for psychosis.
Schizophrenia has also been related to pro-inflammatory status (Goldsmith et al., 2016). The administration of vaccine elicits an immune response including a cellular immune reaction which leads to T-helper cells production of pro-inflammatory cytokines.
The catatonic features in the index case could be due disruption of the associative functions, especially the connectivity of frontal lobes with parietal cortex and motor areas, contributing to the akinetic form of catatonia (Ellul and Choucha, 2015). Though the risk of developing psychosis after covid vaccine is very rare (Reinfeld et al., 2021), medical professionals need to be sensitized about this for early recognition and benefits of the patient.”
β-Amyloid Deposits in Young COVID Patients
Rhodes et al., SSRN, 2022.01
“Cognitive dysfunction, sometimes described as “brain fog”, has become well recognized as a persistent symptom in a substantial fraction of patients who survive SARS-CoV-2 infection [1-3]. Moreover, there is excellent epidemiologic evidence that the risk of a variety of neurologic and psychiatric diseases including dementia is increased among COVID-19 survivors [4]. These are a heterogenous group of patients with a wide variety of comorbidities and there are undoubtably multiple pathophysiologic mechanisms responsible for their neurological symptoms (for review see Nalbandian et al. (2021) [5]). In this report we describe the serendipitous observation of large numbers of focal β-amyloid deposits in the neocortex of autopsied COVID-19 patients. We also found similar deposits in the brains of non- COVID patients who had suffered significant hypoxia, suggesting that this response is hypoxia driven and not specific to a SARS-CoV-2 infection.”
“There is a growing body of literature regarding cognitive problems in post-acute COVID patients ("brain fog"), as well as epidemiologic evidence suggesting that neurocognitive deficits are common in COVID survivors [2-4, 12, 13]. Furthermore, long-term cognitive impairment is common after ARDS [14] and the presence of these deposits in non-COVID brains of patients with ARDS shows that they are not specific to SARS-CoV-2 infections. Rather, it is likely that the deposits correlate with hypoxic damage, since our COVID-19 patients all sustained some degrees of hypoxia and the brains contained widespread hypoxic pathology [6]. This idea is consistent with studies describing a HIF-1α mediated increase in β- amyloid production [15-17] as well as a hypoxia-induced reduction in β-amyloid degradation [18].”
Figure 1: Section of dorsolateral prefrontal cortex of the index patient immunostained with monoclonal 4G8 for beta-amyloid showing numerous diffuse amyloid deposits. The cortical surface is just above the top of the figure and the subcortical white matter is at the bottom of the figure. This section is representative of the entire neocortex. The inset shows a higher magnification view of the boxed area. Note the intracellular cytoplasmic immunostaining (arrows).
Aphasia seven days after second dose of an mRNA-based SARS-CoV-2 vaccine
Finsterer and Korn, Brain Hemorrhages, 2021.12
Case report: “A 52yo male developed sudden-onset reading difficulty and aphasia 7d after the second dose of an mRNA-based SARS-CoV-2 vaccine.
The presented patient is interesting for ICB shortly after the second dose of an mRNA-based SARS-CoV-2 vaccine. Whether there was a causal relation between vaccination and the intracerebral bleeding (ICB) remains speculative. Arguments for a causal relation are that ICB has been reported as a complication of a SARS-CoV-2 vaccination, that ICB occurred time-linked to the vaccination, and that arterial hypertension can be a complication of a SARS-CoV-2 vaccination. A further argument in favour of a causal relation is that ICB has been repeatedly reported as a complication of infections with SARS-CoV-2. Whether the slightly elevated D-dimer indicates hypercoagulability followed by compensatory hypocoagulability remains speculative. Pathophysiological mechanisms explaining SARS-CoV-2 associated ICB could be endothelial cell dysfunction due to direct invasion of the virus, vaccination induced thrombocytopenia, hypocoagulability due to disseminated intravascular coagulopathy or increased fibrinolysis, or arterial hypertension due to affection of the autonomic innervation of the heart, Arguments against a causal relation are that the patient also had slightly elevated arterial hypertension on admission, that the latency between vaccination and ICB was 7 days, and that ICB after SARS-CoV2 vaccination has been only rarely reported. Possibly, the vaccination increased systolic blood pressure, or caused immune-mediated thrombocytopenia or hypocoagulability. The latency of 7 days argues against an immune mechanism as the T-cell response to the vaccination peaks not earlier than 14 days after vaccination.
This case shows that the second dose of a mRNA-based SARS-CoV-2 vaccine may be followed by ICB. Though the pathophysiology of ICB remains unexplained a causal relation between ICB and the vaccination cannot be excluded. Infectiologists and neurologists should remain vigilant for complications of SARS-CoV-2 vaccination. Risk factors for ICB should be carefully monitored in patients undergoing SARS-CoV-2 vaccination”
Fig. 1. cerebral MRI on admission showing an ICB in the left temporal lobe on T2-weighted images (left) and on susceptibility-weighted imaging (SWI) sequencies (right).
Psychiatric and neuropsychiatric sequelae of COVID-19 – A systematic review
Schou et al., Brain, Behavior, and Immunity, 2021.10
Highlights
66 studies examined psychiatric or neuropsychiatric sequelae following COVID-19.
Fatigue, anxiety/depression, PTSD, cognitive deficits, and sleep disturbances were most commonly reported.
COVID-19 survivors were at risk of psychiatric sequelae but symptoms generally improve over time.
“Forty studies reported anxiety and/or depression, 20 studies reported symptoms- or diagnoses of post-traumatic stress disorder (PTSD), 27 studies reported cognitive deficits, 32 articles found fatigue at follow-up, and sleep disturbances were found in 23 studies. Highlighted risk factors were disease severity, duration of symptoms, and female sex. One study showed brain abnormalities correlating with cognitive deficits, and several studies reported inflammatory markers to correlate with symptoms. Overall, the results from this review suggest that survivors of COVID-19 are at risk of psychiatric sequelae but that symptoms generally improve over time.”
SARS-COV-2 Vaccines and Neurodegenerative Disease
Seneff, 2021.06
“There are many reasons to be wary of the COVID-19 vaccines, which have been rushed to market with grossly inadequate evaluation and aggressively promoted to an uninformed public, with the potential for huge, irreversible, negative consequences. One potential consequence is to exhaust the finite supply of progenitor B cells in the bone marrow early in life, causing an inability to mount new antibodies to infectious agents. An even more worrisome possibility is that these vaccines, both the mRNA vaccines and the DNA vector vaccines, may be a pathway to crippling disease sometime in the future. Through the prion-like action of the spike protein, we will likely see an alarming increase in several major neurodegenerative diseases, including Parkinson’s disease, CKD, ALS and Alzheimer’s, and these diseases will show up with increasing prevalence among younger and younger populations, in years to come. Unfortunately, we won’t know whether the vaccines caused this increase, because there will usually be a long time separation between the vaccination event and the disease diagnosis. Very convenient for the vaccine manufacturers, who stand to make huge profits off of our misfortunes — both from the sale of the vaccines themselves and from the large medical cost of treating all these debilitating diseases.”
Zhou et. al, Alzheimer's Research & Therapy, 2021.06
“We found significant network-based relationships between COVID-19 and neuroinflammation and brain microvascular injury pathways and processes which are implicated in Alzheimer’s disease (AD). We also detected aberrant expression of AD biomarkers in the cerebrospinal fluid and blood of patients with COVID-19. While transcriptomic analyses showed relatively low expression of SARS-CoV-2 entry factors in human brain, neuroinflammatory changes were pronounced. In addition, single-nucleus transcriptomic analyses showed that expression of SARS-CoV-2 host factors (BSG and FURIN) and antiviral defense genes (LY6E, IFITM2, IFITM3, and IFNAR1) was elevated in brain endothelial cells of AD patients and healthy controls relative to neurons and other cell types, suggesting a possible role for brain microvascular injury in COVID-19-mediated cognitive impairment. Overall, individuals with the AD risk allele APOE E4/E4 displayed reduced expression of antiviral defense genes compared to APOE E3/E3 individuals.
Our results suggest significant mechanistic overlap between AD and COVID-19, centered on neuroinflammation and microvascular injury.”
Fig. 4. Elevated expression of SARS-CoV-2 host factors in human brain endothelial cells.
a UMAP visualization of the single-nuclei RNA-sequencing dataset from the prefrontal cortex region of Alzheimer’s disease (AD, n = 12) patients and healthy controls (CT, n = 9). b Expression of the entry factors and antiviral defense proteins in different cell types in AD and CT groups. c Network analyses of the antiviral defense genes that are differentially expressed in brain endothelial cells vs. other cell types. Node shape indicates the number of SARS-CoV-2 host factor datasets that contain the node. Edge colors indicate the protein-protein interaction source type. d Expression of the entry factors and antiviral defense proteins in individuals with different APOE genotypes (AD-E3/E3 n = 4, AD-E4/E4 n = 2, AD-E3/E4 n = 5, AD-E2/E4 n = 1, CT-E2/E3 n = 2, CT-E3/E3 n = 5, CT-E3/E4 n = 2). Excit neuron, excitatory neuron. Inhibit neuron, Inhibitory neuron
Yapici-Eser et al., Frontiers in Human Neuroscience, 2021.03
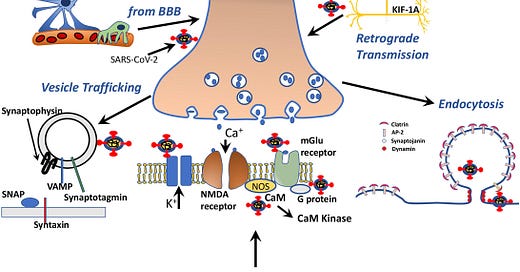
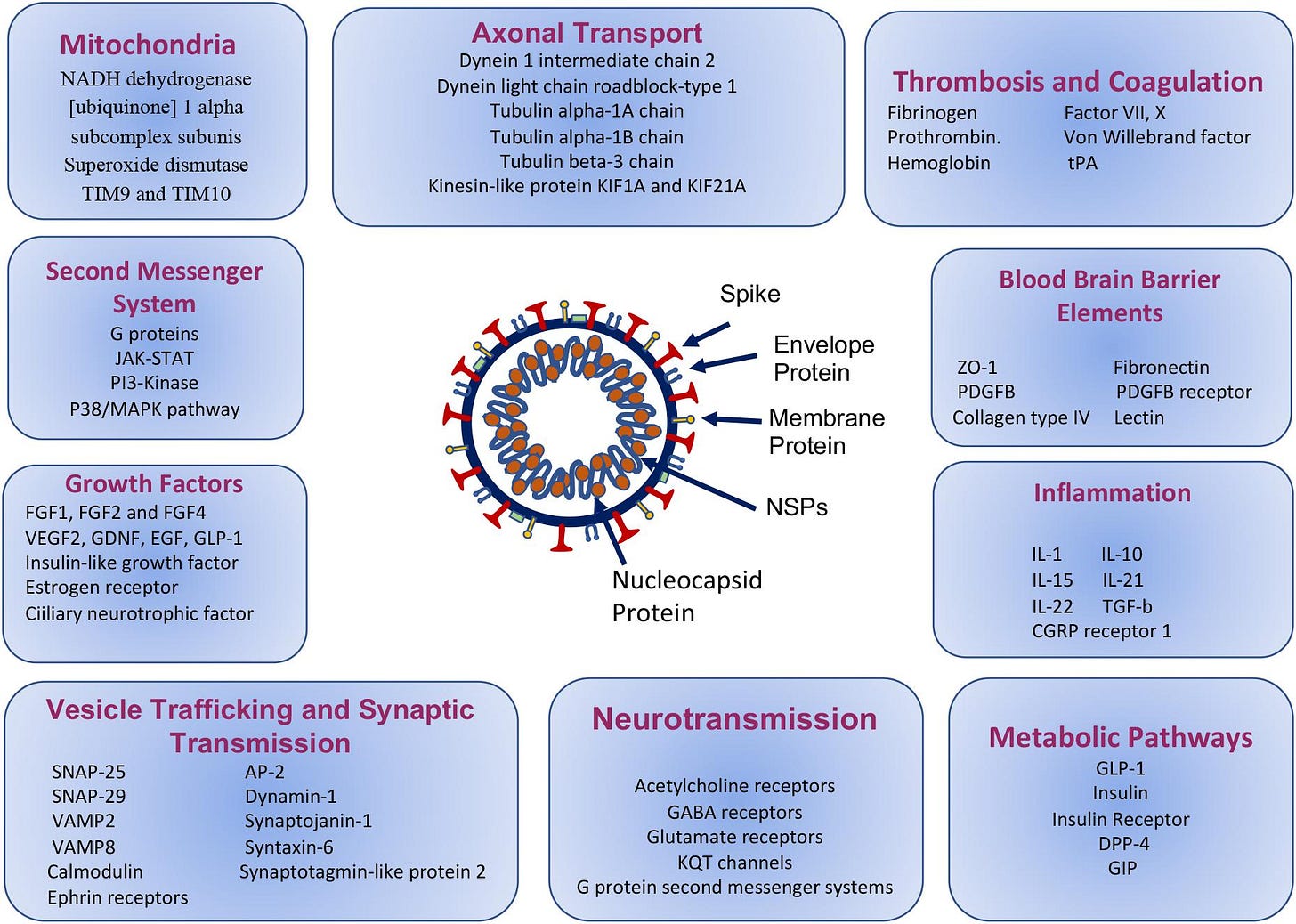
Results: “Predicted Human-SARS-CoV-2 protein interactions have been extensively compared with the literature. Based on the analysis of the molecular functions, cellular localizations and pathways related to human proteins, SARS-CoV-2 proteins are found to possibly interact with human proteins linked to synaptic vesicle trafficking, endocytosis, axonal transport, neurotransmission, growth factors, mitochondrial and blood-brain barrier elements, in addition to its peripheral interactions with proteins linked to thrombosis, inflammation and metabolic control.”
“In conclusion, we represent here the candidate proteins that are most likely to affect the neuropsychiatric representations of COVID-19. SARS-CoV-2 proteins mimic the human protein interactions for blood-brain barrier formation, synaptic vesicle trafficking, endocytosis, axonal transport, neurotransmission, apoptosis and also coagulation, inflammation, and metabolic control, which may result in the development of delirium, psychotic features, seizures, encephalitis, stroke, sensory impairments, peripheric nerve diseases, and autoimmune disorders. With different personal risk factors, different pathways may be triggered, and may cause a different phenotype for COVID-19 clinical presentation. Our findings are also supported by the previous in vivo and in vitro studies from other viruses. Similar studies can be conducted to better understand SARS-CoV-2 pathology. Further in vivo and in vitro studies using the proteins that we pointed to, could pave new targets both for avoiding and reversing neuropsychiatric presentations.”
Figure 3. Molecular paths of COVID-19 associated neuropsychiatric symptoms, based on the mimicry of the selected human protein interactions with SARS-CoV-2 proteins.
Figure 4. Schematic drawing representing SARS-CoV-2 protein-human proteins interactions associated with vesicle transport, neurotransmission, endocytosis and axonal transport.
COVID-19 and neurocognitive disorders
Mukaetova-Ladinska et al., Current Opinion in Psychiatry, 2021.03
Recent findings: “We synthetize the current knowledge of viral (SARS-CoV-2) induced inflammation, mechanisms to viral entry into the central nervous system and altered neurotransmitter systems to provide an informed neurobiological explanation for the rise of neurocognitive disorders (NCD) (defined as per the DSM-5 criteria).
Conclusion: Mild NCD symptoms as a result of the COVID-19 pandemic provide a unique opportunity for researchers to address the early changes that underlie neurocognitive impairment at a clinical and molecular level and to longitudinally follow them with a view to modify their outcomes. From the studies published to date, we know that the biological markers for major NCD of Alzheimer's type develop several decades prior to overt clinical symptoms and it is the convergence of multiple diseases that underpins most clinical dementia syndromes.
The stress related to the COVID-19 pandemic affects similarly the cytokine system and cholinergic pathways, resulting in depression and poor cognitive performance. It is, thus, important to raise awareness for these consequences among the wider population and put into place ways of increasing people's resilience across the lifespan. Identifying biomarkers that may aid in gauging physical and mental resilience will facilitate timely interventions in preparedness for future similar health events.
The properties of SARS-CoV-2 as both a catalyzer and accelerator to brain protein aggregation is another research opportunity for the dementia field in preventing the aggregate prone brain proteins, such as tau protein, β-amyloid and α-synuclein, to form the insoluble and neuronal detrimental deposits. In doing so, we can expect a new generation of therapeutics to be developed, focused on biological mechanisms to prevent and eventually reverse the neurodegenerative processes occurring with ageing and dementia.”
Figure 1 Impact of infective (i.e. SARS-CoV-2) and psychological stressors on the nervous, immune, and endocrine systems. For a more detailed explanation, see the main text. NCD, neurocognitive disorder; OCD, obsessive compulsive disorder; PTSD, post-traumatic stress disorder.
Gennaro et al., Brain Behavior and Immunity, 2021.02
“COVID-19 outbreak is associated with mental health implications during viral infection and at short-term follow-up. …
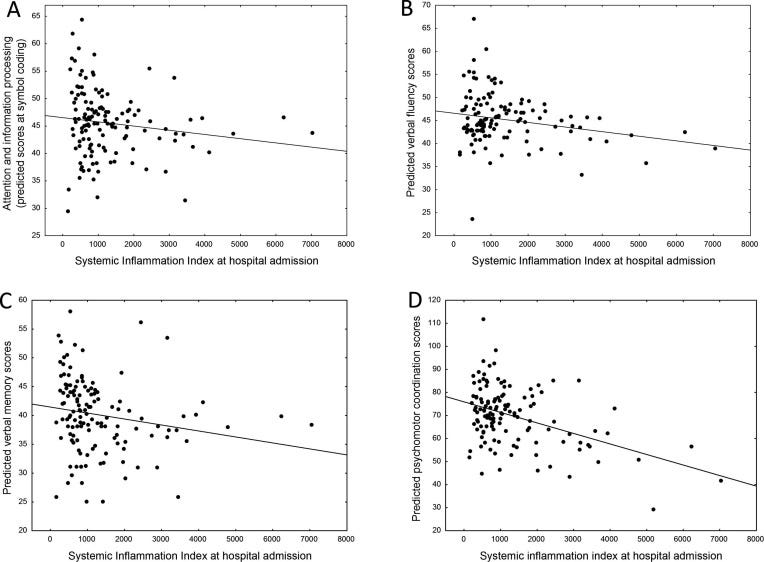
Three months after discharge from the hospital, 35.8% still self-rated symptoms in the clinical range in at least one psychopathological dimension. We observed persistent depressive symptomatology, while PTSD, anxiety, and insomnia decreased during follow-up. Sex, previous psychiatric history, and the presence of depression at one month affected the depressive symptomatology at three months. Regardless of clinical physical severity, 78% of the sample showed poor performances in at least one cognitive domain, with executive functions and psychomotor coordination being impaired in 50% and 57% of the sample.
Baseline systemic immune-inflammation index (SII), which reflects the immune response and systemic inflammation based on peripheral lymphocyte, neutrophil, and platelet counts, predicted self-rated depressive symptomatology and cognitive impairment at three-months follow-up; and changes of SII predicted changes of depression during follow-up. Neurocognitive impairments associated with severity of depressive psychopathology, and processing speed, verbal memory and fluency, and psychomotor coordination were predicted by baseline SII.
We hypothesize that COVID-19 could result in prolonged systemic inflammation that predisposes patients to persistent depression and associated neurocognitive dysfunction. The linkage between inflammation, depression, and neurocognition in patients with COVID-19 should be investigated in long-term longitudinal studies, to better personalize treatment options for COVID-19 survivors.”
Fig. 2. Effect of systemic inflammation (SII) at hospital admission, on neurocognitive performances at three-month follow-up as measured with BACS. A: Attention and speed of information processing (symbol coding). B: Verbal fluency. C: Verbal memory. D: Psychomotor coordination.
Cerebral Micro-Structural Changes in COVID-19 Patients – An MRI-based 3-month Follow-up Study
Lu et al., EClinicalMedicine, 2020.08
Abstract: “Increasing evidence supported the possible neuro-invasion potential of SARS-CoV-2. In this prospective study, diffusion tensor imaging (DTI) and 3D high-resolution T1WI sequences were acquired in 60 recovered COVID-19 patients.
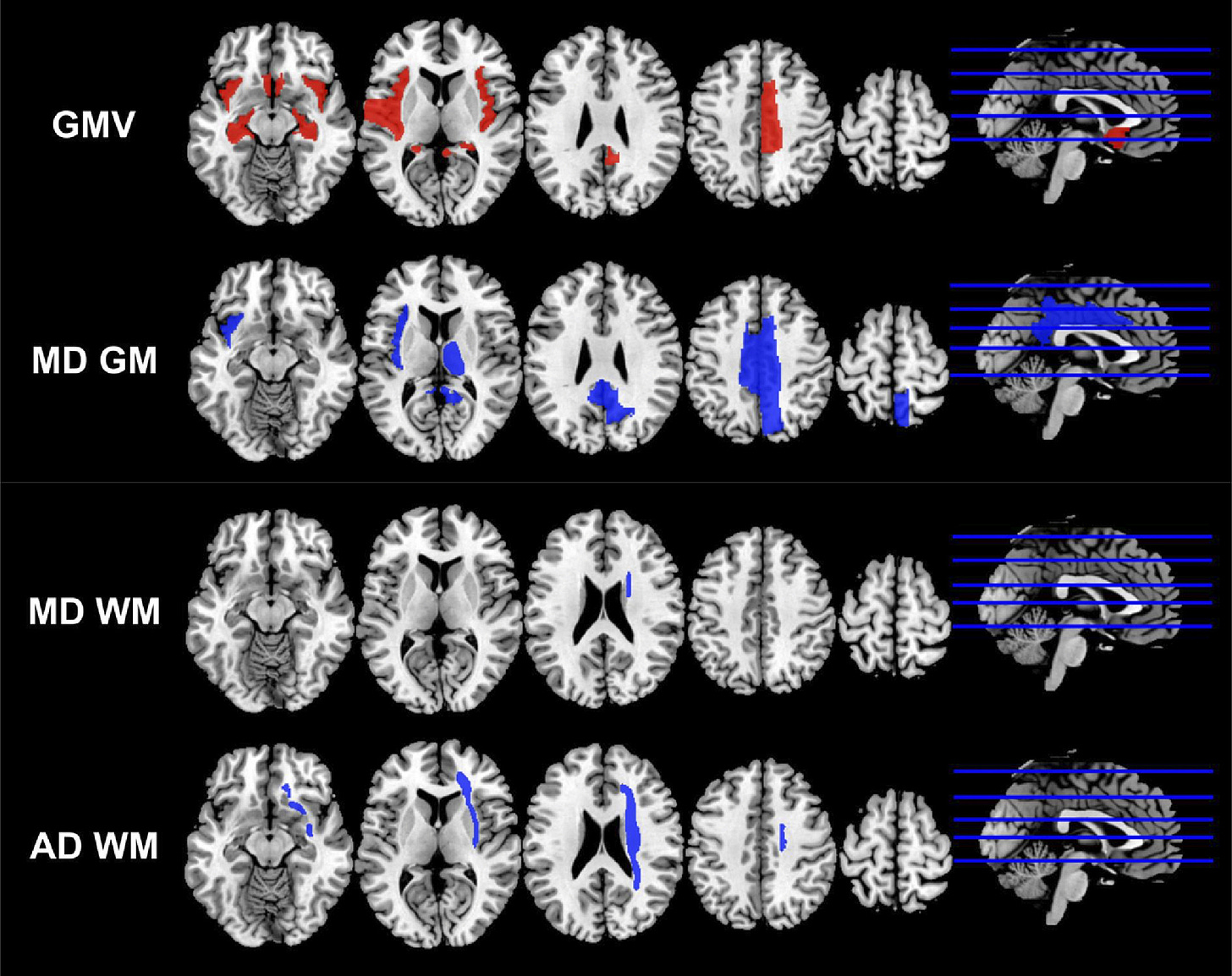
Findings: In this follow-up stage, neurological symptoms were presented in 55% COVID-19 patients. COVID-19 patients had statistically significantly higher bilateral gray matter volumes (GMV) in olfactory cortices, hippocampi, insulas, left Rolandic operculum, left Heschl's gyrus and right cingulate gyrus and a general decline of MD, AD, RD accompanied with an increase of FA in white matter, especially AD in the right CR, EC and SFF, and MD in SFF compared with non-COVID-19 volunteers (corrected p value <0.05). Global GMV, GMVs in left Rolandic operculum, right cingulate, bilateral hippocampi, left Heschl's gyrus, and Global MD of WM were found to correlate with memory loss (p value <0.05). GMVs in the right cingulate gyrus and left hippocampus were related to smell loss (p value <0.05). MD-GM score, global GMV, and GMV in right cingulate gyrus were correlated with LDH level (p value <0.05).
Interpretation: Study findings revealed possible disruption to micro-structural and functional brain integrity in the recovery stages of COVID-19, suggesting the long-term consequences of SARS-CoV-2.
Discussion: It is important to investigate the relationship between abnormal anatomical brain areas and ACE-2 distribution. It is clarified that SARS-CoV-2 enters the host cell by attaching with ACE-2 via Spike (S) glycoprotein. Therefore, the more expression of ACE-2 might bring more severe abnormalities. The distribution of ACE2 was non-equivalent over the brain and was most frequently expressed in substantia nigra, followed by spinal cord, hippocampus, basal ganglia, limbic system and frontal cortex [[17]]. Our results suggested that various components in the limbic system were affected structures sharing possible high ACE-2 expression, which were partly aligned with the proposed ACE-2-riched regions. Although we were not able to observe the DTI metrics in substantia nigra since it was not included in the brain atlas we used, it was still very hard to support any relationship between ACE2 expression and affected brain areas.
In this prospective study, volumetric and micro-structural abnormalities were detected mainly in the central olfactory cortices, partial white matter in the right hemisphere from recovered COVID-19 patients, providing new evidence to the neurological damage of SARS-CoV-2. The abnormalities in these brain areas might cause long-term burden to COVID-19 patients after recovery.”
Figure 2 The regions with statistically significant differences in the volumes and diffusion indices of the COVID-19 group compared with the control group. The regions with relative higher mean values in the COVID-19 group were marked as red, and the regions with relative lower mean values in the COVID-19 group were marked as blue. GMV: gray matter volume; MD GM: mean diffusivity of gray matter; MD WM: mean diffusivity of white matter; AD WM: axial diffusivity of white matter.