Brain Facts - A Primer on the Brain & Nervous System, SFN, BrainFacts.org
Principles of Neural Science, (5th Ed. pdf)
Neuroscience posters: Neurodegeneration, Neuroinflammation
Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates
Rutkai et al., Nature Communications, 2022.04
“Neurological manifestations are a significant complication of coronavirus disease (COVID-19), but underlying mechanisms aren’t well understood. The development of animal models that recapitulate the neuropathological findings of autopsied brain tissue from patients who died from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are critical for elucidating the neuropathogenesis of infection and disease. Here, we show neuroinflammation, microhemorrhages, brain hypoxia, and neuropathology that is consistent with hypoxic-ischemic injury in SARS-CoV-2 infected non-human primates (NHPs), including evidence of neuron degeneration and apoptosis. Importantly, this is seen among infected animals that do not develop severe respiratory disease, which may provide insight into neurological symptoms associated with “long COVID”. Sparse virus is detected in brain endothelial cells but does not associate with the severity of central nervous system (CNS) injury. We anticipate our findings will advance our current understanding of the neuropathogenesis of SARS-CoV-2 infection and demonstrate SARS-CoV-2 infected NHPs are a highly relevant animal model for investigating COVID-19 neuropathogenesis among human subjects.”
Fig. 2: Neuronal pathology and cell death in SARS-CoV-2 infection.
Representative H&E images show a healthy Purkinje cell layer in the cerebellum of a non-infected control RM6 (a) and reveal cell death-associated neuronal changes in cerebellum of infected animals, (AGM4 b, AGM3 c), and brainstem from AGM3 (d). Arrows indicate pyknotic and karyolitic Purkinje cells and cellular blebs. Asterisks denote areas of tissue necrosis/vacuolation on H&E sections (b) and (d). H&E was performed and assessed twice on all brain regions. Neuronal degeneration in cerebellum was only seen in the context of infection, visualized by positive, green FluoroJade C-stained neurons (AGM2 e, AGM1 f). FluoroJade C staining was performed twice on the brain regions investigated. Abnormal neuronal morphology and cleaved caspase 3 positivity is demonstrated in cerebellum (AGM3 g) and brainstem (RM2 h). Summary data of cleaved caspase 3 positive cells stratified by brain region (i) where n = 4 biologically independent samples/brain region in the control group and n = 8 biologically independent samples/brain region in the infected group, except OL where n = 7 infected animals. Immunohistochemical staining for cleaved caspase 3 was performed twice on all brain regions. Statistics were performed with a two-tailed Mann–Whitney U test. *p ≤ 0.05 and **p ≤ 0.005 comparing mock-infected to infected animals. Data are expressed as mean ± SEM. p values: CER = 0.0081, BS = 0.0182, FL = 0.0323, OL = 0.0061, TL = 0.0485, PL = 0.0485, BG = 0.0040 (control vs. infected). Source data are provided as a Source Data file. Abbreviations: CER cerebellum, BS brainstem, FL frontal lobe, OL occipital lobe, TL temporal lobe, PL parietal lobe, BG basal ganglia, C control, I infected, AGM African green monkey, RM Rhesus macaque. Scale bars = 100 µm (a, c–f, h) and 50 µm (b, g).
Brain cortical changes are related to inflammatory biomarkers in hospitalized SARS-CoV-2 patients with neurological symptoms
Sanabria-Diaz et al., medRxiv, 2022.02
“Increasing evidence shows that the brain is a target of SARS-CoV-2. However, the consequences of the virus on the cortical regions of hospitalized patients are currently unknown. The purpose of this study was to assess brain cortical gray matter volume (GMV), thickness (Th), and surface area (SA) characteristics in SARS-CoV-2 hospitalized patients with a wide range of neurological symptoms and their association with clinical indicators of inflammatory processes. A total of 33 patients were selected from a prospective, multicenter, cross-sectional study during the ongoing pandemic (August 2020-April 2021) at Basel University Hospital. Retrospectively biobank healthy controls with the same image protocol served as controls group. For each anatomical T1w MPRAGE image, the Th and GMV segmentation were performed with the FreeSurfer-5.0. Cortical measures were compared between groups using a linear regression model. The covariates were age, gender, age*gender, MRI magnetic field strength, and total intracranial volume/mean Th/Total SA. The association between cortical features and laboratory variables was assessed using partial correlation adjusting for the same covariates. P-values were adjusted using false discovery rate (FDR). Our findings revealed a lower cortical gray matter volume in orbitofrontal and cingulate regions in patients compared to controls. The orbitofrontal grey matter volume was negatively associated with protein levels, CSF-blood/albumin ratio and CSF EN-RAGE level. CSF EN-RAGE and CSF/Blood-albumin ratio, which are neuroinflammatory biomarkers, were associated with cortical alterations in gray matter volume and thickness in frontal, orbitofrontal, and temporal regions. Our data suggest that viral-triggered inflammation leads to increased neurotoxic damage in some cortical areas.”
Fig 1. Map of the brain regions with a significant association between gray matter volume and cortical thickness clinical variables in severly affected SARS-CoV-2 patients. Panel A) show the 27 brain regions with significant correlation values for gray matter volume and cortical thickness after multiple comparison corrections (False Discovery Rate-FDR). Panel B) and C) shows the matrices representing the association significance (significant p-corrected<0.05 in red squares). CSF=Cerebrospinal fluid, leuk=leukocytes, lact=lactate, prot=protein, albR=Albumin CSF-blood ratio, Cen_Rage: CSF EN-RAGE = extracellular receptor for advanced glycation end-products binding protein, R= right, L= left.
The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2
Krasemann et al., Stem Cell Reports, 2022.02
“Neurological complications are common in COVID-19. Although SARS-CoV-2 has been detected in patients’ brain tissues, its entry routes and resulting consequences are not well understood. Here, we show a pronounced upregulation of interferon signaling pathways of the neurovascular unit in fatal COVID-19. By investigating the susceptibility of human induced pluripotent stem cell (hiPSC)-derived brain capillary endothelial-like cells (BCECs) to SARS-CoV-2 infection, we found that BCECs were infected and recapitulated transcriptional changes detected in vivo. While BCECs were not compromised in their paracellular tightness, we found SARS-CoV-2 in the basolateral compartment in transwell assays after apical infection, suggesting active replication and transcellular transport of virus across the blood-brain barrier (BBB) in vitro. Moreover, entry of SARS-CoV-2 into BCECs could be reduced by anti-spike-, anti-angiotensin-converting enzyme 2 (ACE2)-, and anti-neuropilin-1 (NRP1)-specific antibodies or the transmembrane protease serine subtype 2 (TMPRSS2) inhibitor nafamostat. Together, our data provide strong support for SARS-CoV-2 brain entry across the BBB resulting in increased interferon signaling.”
COVID-19 infection enhances susceptibility to oxidative-stress induced parkinsonism
Smeyne et al., bioRvix, 2022.02
Objectives: “To determine if prior infection with SARS-CoV-2 increased sensitivity to a mitochondrial toxin known to induce parkinsonism.
Methods: hACE2 mice were infected with SARS-CoV-2 to induce mild to moderate disease. After 31 days recovery, mice were administered a non-lesion inducing dose of the parkinsonian toxin MPTP. Subsequent neuroinflammation and SNpc dopaminergic neuron loss was determined and compared to SARS-CoV-2 or MPTP alone.
Results: hACE2 mice infected with SARS-CoV-2 or MPTP showed no SNpc DA neuron loss following MPTP. In mice infected and recovered from SARS-CoV-2 infection, MPTP induced a 23% or 19% greater loss of SNpc dopaminergic neurons than SARS-CoV-2 or MPTP, respectively (p < 0.05). Examination of microglial activation showed a significant increase in the number of activated microglia in the SARS-CoV-2 + MPTP group compared to SARS-CoV-2 or MPTP alone.”
Figure 1. Effect of SARS-CoV-2 infection on sensitivity to subacute MPTP infection.
A. Kaplan- Meier Survival curve of mice infected with 3 different titers of SARS-CoV-2 (USA-1). B. Antibody titers in mice infected 45 days prior to testing. C. Number of SNpc dopaminergic neurons in the SNpc. D. Total number of microglia in the SNpc. E. Total number of resting microglia in the SNpc. F. Total number of activated microglia in the SNpc. G. Low power (4x) micrograph showing appearance of the dopaminergic neurons (TH+ neurons) in the rostral tier of the substantia nigra pars compacta. The SARS + MPTP group has a significant loss of these neurons as well as its fibers as noted by the decreased TH-immunoreactivity. H. Higher power photomicrograph (40x) of the same sections shown in G. Resting microglia are small and have thin processes and are labeled with blue arrowheads. Activated microglia have a larger cell body, and thickened processes; labeled by red arrows. Statistical analysis was performed using ANOV A with post-hoc tests (Tukey) (Prism 9.0, GraphPad Software) if overall significance was achieved.
NRP1 and furin as putative mediators of SARS-CoV-2 entry into human brain cells
Kumar et al., bioRxiv, 2022.01
“COVID-19 has prominent neurological manifestations including psychiatric symptoms, indicating significant synaptic pathology. Surprisingly, existing evidence suggests negligible expression of the key SARS-CoV-2 host cell entry mediators ACE2 and TMPRSS2 in human brain, which complicates understanding of the pathomechanisms of the neuropsychiatric manifestations in COVID-19. Recent studies suggested that an alternative host-cell entry receptor, NRP1, can mediate entry of furin cleaved SARS-CoV-2 spike proteins into the host cells. However, the role of NRP1 and furin in mediating SARS-CoV-2 entry in human brain cells has been least explored and remains a lacuna in the literature. We performed an in silico analysis of the transcriptomic and proteomic expressions of SARS-CoV-2 host-cell entry receptors and associated tissue proteases in human brain tissue, using the publically available databases. Based on the expression analysis, SARS-CoV-2 entry in human brain cells is likely to be mediated through NRP1 and furin.”
“COVID-19 Vaccines & neurodegenerative disease”, Dr. S. Seneff, 2022.01.03, 📹
Seneff and Nigh, Int. Journal of Vaccine Theory, Practice, & Research, 2021.05
Fernández-Castañeda et al., bioRxiv, 2022.01
“Survivors of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection frequently experience lingering neurological symptoms, including impairment in attention, concentration, speed of information processing and memory. This long-COVID cognitive syndrome shares many features with the syndrome of cancer therapy-related cognitive impairment (CRCI). Neuroinflammation, particularly microglial reactivity and consequent dysregulation of hippocampal neurogenesis and oligodendrocyte lineage cells, is central to CRCI. We hypothesized that similar cellular mechanisms may contribute to the persistent neurological symptoms associated with even mild SARS-CoV-2 respiratory infection. Here, we explored neuroinflammation caused by mild respiratory SARS-CoV-2 infection – without neuroinvasion - and effects on hippocampal neurogenesis and the oligodendroglial lineage. Using a mouse model of mild respiratory SARS-CoV-2 infection induced by intranasal SARS-CoV-2 delivery, we found white matter-selective microglial reactivity, a pattern observed in CRCI. Human brain tissue from 9 individuals with COVID-19 or SARS-CoV-2 infection exhibits the same pattern of prominent white matter-selective microglial reactivity. In mice, pro-inflammatory CSF cytokines/chemokines were elevated for at least 7-weeks post-infection; among the chemokines demonstrating persistent elevation is CCL11, which is associated with impairments in neurogenesis and cognitive function. Humans experiencing long-COVID with cognitive symptoms (48 subjects) similarly demonstrate elevated CCL11 levels compared to those with long-COVID who lack cognitive symptoms (15 subjects). Impaired hippocampal neurogenesis, decreased oligodendrocytes and myelin loss in subcortical white matter were evident at 1 week, and persisted until at least 7 weeks, following mild respiratory SARS-CoV-2 infection in mice. Taken together, the findings presented here illustrate striking similarities between neuropathophysiology after cancer therapy and after SARS-CoV-2 infection, and elucidate cellular deficits that may contribute to lasting neurological symptoms following even mild SARS-CoV-2 infection.”
Fig. 3 Microglial reactivity in human white matter after SARS-CoV-2 infection
(A) Representative micrographs of CD68 immunostaining (brown) in the gray (cerebral cortex) or subcortical white matter of human subjects with COVID or in non-COVID control subjects. (B) Activated microglia (CD68+ cells) quantification. n=5 for non-COVID control group, n=9 for COVID group.
Data shown as mean +/- SEM; ns p>0.05 by 2-way ANOVA with multiple comparisons; each dot represents a human subject. Scale bars 100μm.
Albornoz et al., bioRxiv, 2022.01
“Coronavirus disease-2019 (COVID-19) is primarily a respiratory disease, however, an increasing number of reports indicate that SARS-CoV-2 infection can also cause severe neurological manifestations, including precipitating cases of probable Parkinson’s disease. As microglial NLRP3 inflammasome activation is a major driver of neurodegeneration, here we interrogated whether SARS-CoV-2 can promote microglial NLRP3 inflammasome activation utilising a model of human monocyte-derived microglia. We identified that SARS-CoV-2 isolates can bind and enter microglia, triggering inflammasome activation in the absence of viral replication. Mechanistically, microglial NLRP3 could be both primed and activated with SARS-CoV-2 spike glycoprotein in a NF-κB and ACE2-dependent manner. Notably, virus- and spike protein-mediated inflammasome activation in microglia was significantly enhanced in the presence of α-synuclein fibrils, which was entirely ablated by NLRP3-inhibition. These results support a possible mechanism of microglia activation by SARS-CoV-2, which could explain the increased vulnerability to developing neurological symptoms akin to Parkinson’s disease in certain COVID-19 infected individuals, and a potential therapeutic avenue for intervention.”
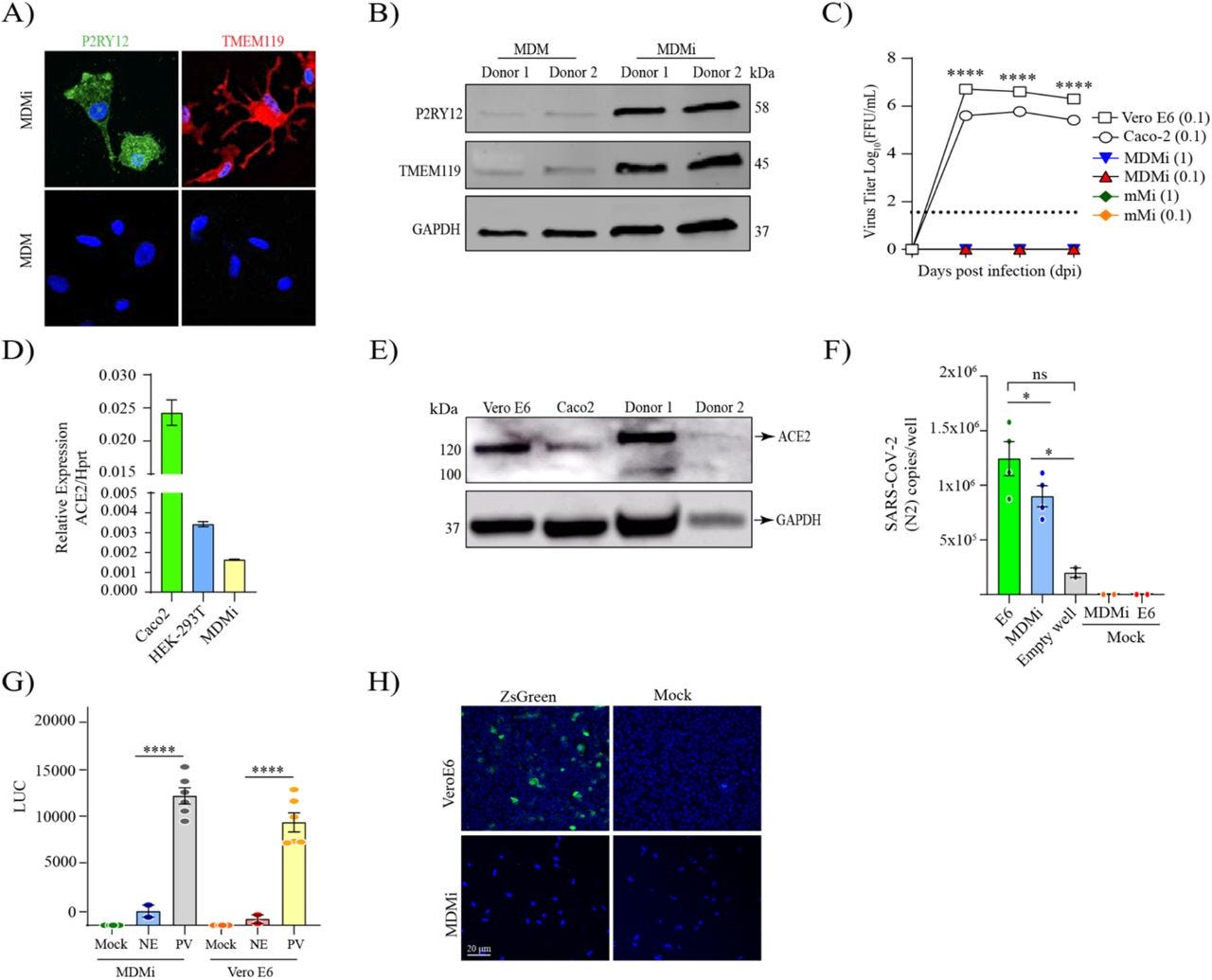
Fig. 1. SARS-CoV-2 isolates can enter human monocyte-derived microglia (MDMi) in the absence of viral replication.
Microglia signature markers, P2RY12 and TMEM119 (in green) and cells nuclei (in blue) assessed by immunofluorescence staining, and representative western blot are presented in panel (A-B), respectively. Growth kinetics of SARS-CoV-2 (D614) on MDMi, mouse microglia (mMi), Vero E6 and Caco2 cells in (C). Relative expression of ACE2 in MDMi by qPCR compared to Vero E6 and Hek-293T in (D). Level of ACE2 receptor in MDMi and mouse microglia compared to Caco2 and Vero E6 cells analysed by western blot shown in panel (E). Viral RNA levels from SARS-CoV-2 particles bound on cell surface expressed as N2 copies/well in (F) Intracellular luciferase level (LUC) delivered by pseudo-virus (PV) particle for SARS-CoV-2 in MDMi and Vero E6 compared to the non-glycoprotein control (NE) in (G). SARS-CoV-2 replication on MDMi (at MOI of 1) and Vero E6 (at MOI of 0.01) using SARS-CoV-2 reporter virus expressing ZsGreen fluorescent protein assessed directly under confocal microscopy at 3dpi are shown in panel (H). Data points are means + SEM from at least three different donors. *P < 0.05, **P < 0.01, and ***P < 0.001 and **** P < 0.0001 by two-way ANOVA test with Sidak’s correction.
Could Small Neurotoxins-Peptides be Expressed during SARS-CoV-2 Infection?
Cafiero et al., Current Genomics, 2021.12
“SARS-CoV-2 pathogenesis has been recently extended to human central nervous system (CNS), in addition to nasopharyngeal truck, eye, lung and gut. The recent literature highlights that some SARS-CoV-2 spike glycoprotein regions homologous to neurotoxin-like peptides might bind to human nicotinic Acetyl-Choline Receptors (nAChRs). Spike-nAChR interaction can probably cause dysregulation of CNS and cholinergic anti-inflammatory pathways and uncontrolled immune-response, both associated to a severe COVID-19 pathophysiology. Herein, we hypothesize that inside the Open Reading Frame (ORF) region of spike glycoprotein, the RNA polymerase can translate small neurotoxic peptides by means of a “jumping mechanism” already demonstrated in other coronaviruses. These small peptides can bind the snAChRs instead of Spike glycoproteins. A striking homology occurred between these small peptides observed by sequence retrieval and proteins alignment. Acting as nAChRs antagonists, these small peptides (conotoxins) could be the explanation for the extrapulmonary clinical manifestations (neurological, hemorrhagic and thrombotic expressions, the prolonged apnea, the cardiocirculatory collapse, the heart arrhythmias, the ventricular tachycardia, the body temperature alteration, the electrolyte K+ imbalance and finally the significant reduction of butyryl cholinesterase (BuChE) plasma levels, as observed in COVID-19 patients. Several factors might induce the expression of these small peptides, including microbiota. The main hypothesis regarding the presence of these small peptides opens a new scenario on the etiology of COVID-19 clinical symptoms observed so far, including the neurological manifestations.”
Infection of Brain Pericytes Underlying Neuropathology of COVID-19 Patients
Bocci et al., International Journal of Molecular Sciences, 2021.10
“A wide range of neurological manifestations have been associated with the development of COVID-19 following SARS-CoV-2 infection. However, the etiology of the neurological symptomatology is still largely unexplored. Here, we used state-of-the-art multiplexed immunostaining of human brains (n = 6 COVID-19, median age = 69.5 years; n = 7 control, median age = 68 years) and demonstrated that expression of the SARS-CoV-2 receptor ACE2 is restricted to a subset of neurovascular pericytes. Strikingly, neurological symptoms were exclusive to, and ubiquitous in, patients that exhibited moderate to high ACE2 expression in perivascular cells. Viral dsRNA was identified in the vascular wall and paralleled by perivascular inflammation, as signified by T cell and macrophage infiltration. Furthermore, fibrinogen leakage indicated compromised integrity of the blood–brain barrier. Notably, cerebrospinal fluid from additional 16 individuals (n = 8 COVID-19, median age = 67 years; n = 8 control, median age = 69.5 years) exhibited significantly lower levels of the pericyte marker PDGFRβ in SARS-CoV-2-infected cases, indicative of disrupted pericyte homeostasis. We conclude that pericyte infection by SARS-CoV-2 underlies virus entry into the privileged central nervous system space, as well as neurological symptomatology due to perivascular inflammation and a locally compromised blood–brain barrier.”
Figure 2. Perivascular infection by SARS-CoV-2 is paralleled by perivascular inflammation in the brain of COVID-19 patients. (A) Immunohistochemical detection of viral components in COVID-19-infected patients and non-COVID-19 controls. Cell nuclei are counterstained with hematoxylin (blue). The black arrows indicate the chromogenic deposition of the 3,3’-diaminobenzidine (DAB) substrate. (B) Representative field of a 7-plex mIHC staining panel of placental tissue infected with SARS-CoV-2. The magenta arrows indicate accumulation of viral dsRNA in correspondence of the ACE2-positive areas by the specialized epithelial layer of syncytiotrophoblast in the placenta. The intensity of each OPAL fluorophore is further presented in individual photomicrographs. (C) Immunohistochemical detection of dsRNA in the cerebral cortex of a COVID-19 patient and in a non-COVID-19 control. Cell nuclei are counterstained with hematoxylin (blue). Black arrows indicate deposition of the DAB substrate. (D) Composite mIHC image of the perivascular immune cell infiltration in the frontal cortex of a COVID-19 patient and in a control individual. The antibody panel was designed for the concomitant detection of CD34 (endothelium) and five immune cell markers: CD4 (T helper cells), CD8 (cytotoxic T lymphocytes), CD20 (B cells), CD68 (macrophages), and FOXP3 (regulatory T cells).
Spectrum of neurological complications following COVID-19 vaccination
Garg and Paliwal, Neurological Sciences, 2021.10
Conclusion: Post-authorization, a wide spectrum of serious neurological complications has been reported following COVID-19 vaccination. The most devastating neurological complication is cerebral venous sinus thrombosis that has been reported in females of childbearing age following adenovector-based vaccines. Another major neurological complication of concern is Bell’s palsy that was reported dominantly following mRNA vaccine administration. Transverse myelitis, acute disseminated encephalomyelitis, and Guillain-Barré syndrome are other severe unexpected post-vaccination complications that can occur as result of molecular mimicry and subsequent neuronal damage. Most of other serious neurological complications are reported in either in form of isolated case reports or small cases series. A causal association of these adverse events is controversial; large collaborative prospective studies are needed to prove causality.
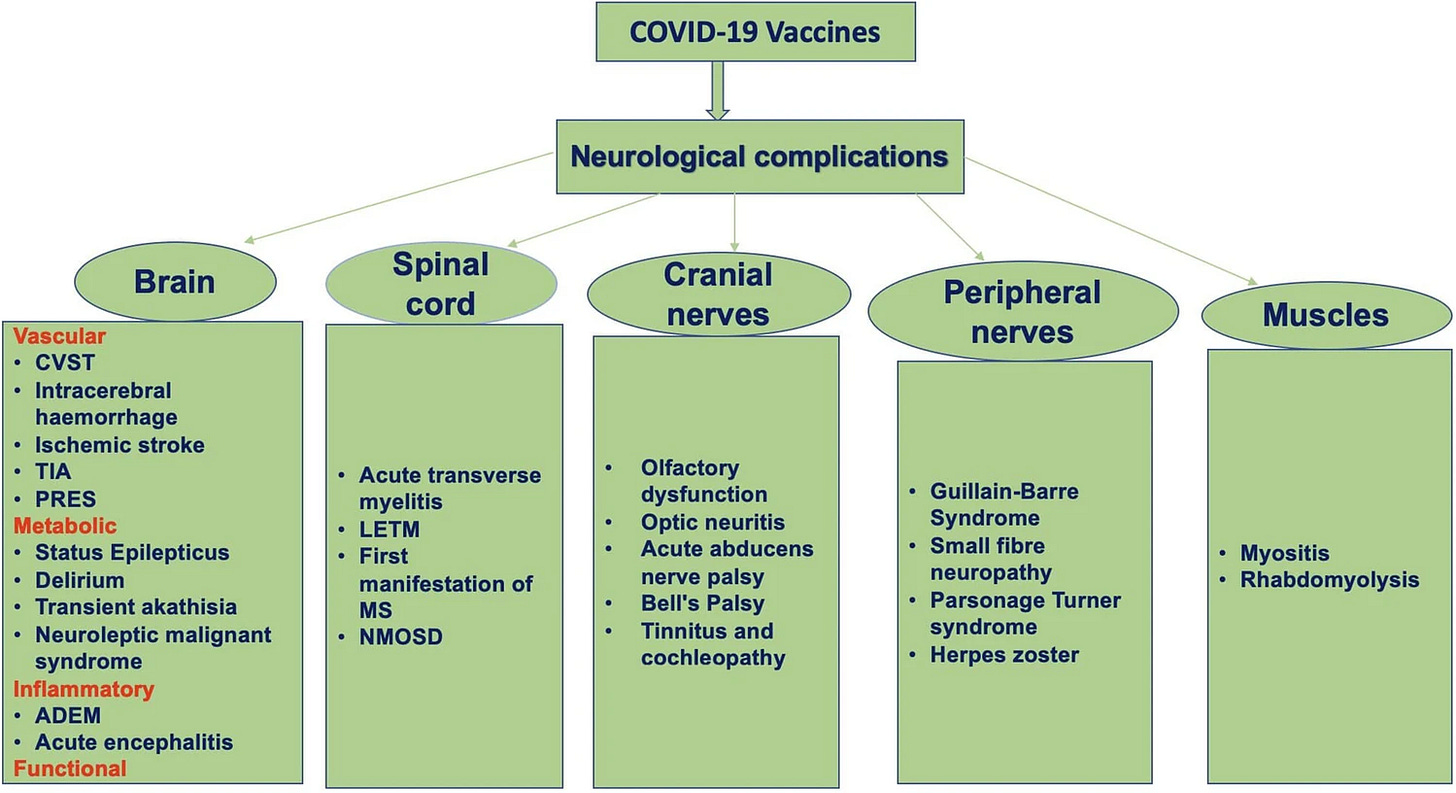
Fig. 1. A flow diagram depicts the spectrum of severe neurological complications following COVID-19 vaccinations (ADEM, acute disseminated encephalomyelitis; CVST, cerebral venous sinus thrombosis; LETM, longitudinally extensive transverse myelitis; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders; PRES, posterior reversible encephalopathy syndrome; TIA, transient ischemic attacks)
SARS-COV-2 Vaccines and Neurodegenerative Disease
S. Seneff, PhD, 2021.06
“There are many reasons to be wary of the COVID-19 vaccines, which have been rushed to market with grossly inadequate evaluation and aggressively promoted to an uninformed public, with the potential for huge, irreversible, negative consequences. One potential consequence is to exhaust the finite supply of progenitor B cells in the bone marrow early in life, causing an inability to mount new antibodies to infectious agents. An even more worrisome possibility is that these vaccines, both the mRNA vaccines and the DNA vector vaccines, may be a pathway to crippling disease sometime in the future. Through the prion-like action of the spike protein, we will likely see an alarming increase in several major neurodegenerative diseases, including Parkinson’s disease, CKD, ALS and Alzheimer’s, and these diseases will show up with increasing prevalence among younger and younger populations, in years to come. Unfortunately, we won’t know whether the vaccines caused this increase, because there will usually be a long time separation between the vaccination event and the disease diagnosis. Very convenient for the vaccine manufacturers, who stand to make huge profits off of our misfortunes — both from the sale of the vaccines themselves and from the large medical cost of treating all these debilitating diseases.”
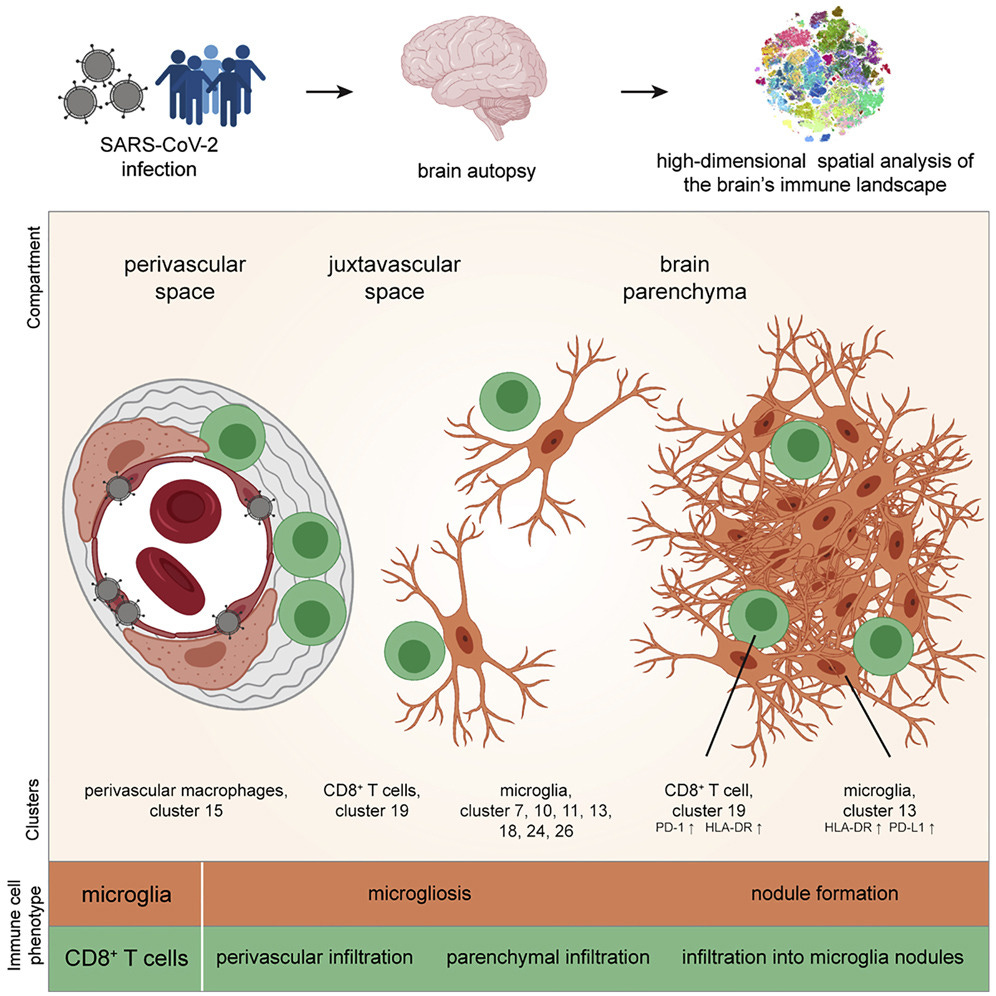
Schwabenland et al., Immunity, 2021.06
“COVID-19 can cause severe neurological symptoms, but the underlying pathophysiological mechanisms are unclear. Here, we interrogated the brain stems and olfactory bulbs in postmortem patients who had COVID-19 using imaging mass cytometry to understand the local immune response at a spatially resolved, high-dimensional, single-cell level and compared their immune map to non-COVID respiratory failure, multiple sclerosis, and control patients. We observed substantial immune activation in the central nervous system with pronounced neuropathology (astrocytosis, axonal damage, and blood-brain-barrier leakage) and detected viral antigen in ACE2-receptor-positive cells enriched in the vascular compartment. Microglial nodules and the perivascular compartment represented COVID-19-specific, microanatomic-immune niches with context-specific cellular interactions enriched for activated CD8+ T cells. Altered brain T-cell-microglial interactions were linked to clinical measures of systemic inflammation and disturbed hemostasis. This study identifies profound neuroinflammation with activation of innate and adaptive immune cells as correlates of COVID-19 neuropathology, with implications for potential therapeutic strategies.”
Harapan and Yoo, Journal of Neurology, 2021.01
“Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus, is responsible for the outbreak of coronavirus disease 19 (COVID-19) and was first identified in Wuhan, China in December 2019. It is evident that the COVID-19 pandemic has become a challenging world issue. Although most COVID-19 patients primarily develop respiratory symptoms, an increasing number of neurological symptoms and manifestations associated with COVID-19 have been observed. In this narrative review, we elaborate on proposed neurotropic mechanisms and various neurological symptoms, manifestations, and complications of COVID-19 reported in the present literature. For this purpose, a review of all current published literature (studies, case reports, case series, reviews, editorials, and other articles) was conducted and neurological sequelae of COVID-19 were summarized. Essential and common neurological symptoms including gustatory and olfactory dysfunctions, myalgia, headache, altered mental status, confusion, delirium, and dizziness are presented separately in sections. Moreover, neurological manifestations and complications that are of great concern such as stroke, cerebral (sinus) venous thrombosis, seizures, meningoencephalitis, Guillain–Barré syndrome, Miller Fisher syndrome, acute myelitis, and posterior reversible encephalopathy syndrome (PRES) are also addressed systematically. Future studies that examine the impact of neurological symptoms and manifestations on the course of the disease are needed to further clarify and assess the link between neurological complications and the clinical outcome of patients with COVID-19. To limit long-term consequences, it is crucial that healthcare professionals can early detect possible neurological symptoms and are well versed in the increasingly common neurological manifestations and complications of COVID-19.”
Is COVID-19 a Perfect Storm for Parkinson’s Disease?
Brundin, Nath, and Beckham, Trends in Neurosciences, 2020.12
We propose three potential mechanisms for the rapid development of parkinsonism following SARS-CoV-2 infection (Figure 1). These mechanisms may operate either alone or in concert.
First, vascular insults have been reported to develop in multiple organs, including the brain, in severe COVID-19 in conjunction with a hypercoagulable state [7.]. This could conceivably directly damage the nigrostriatal system, akin to what is seen in vascular parkinsonism. However, the aforementioned recent autopsy study [6.] did not report any bleeding or small vessel thrombosis in the brain.
Second, considering the association between inflammatory disorders and elevated PD risk, it is possible that marked systemic inflammation caused by severe COVID-19 could trigger neuroinflammation and demise of nigral dopamine neurons. Midbrain dopamine neurons are believed to be particularly susceptible to systemic inflammation. Several studies have demonstrated elevated interleukin (IL)-6 levels in COVID-19, and one report suggested that the kynurenine pathway is perturbed [8.]. Interestingly, these are both mechanisms that have been associated with PD [2.,9.].
Third, SARS-CoV-2 may be a neurotropic virus, because viral RNA has been detected in postmortem brains of some patients with COVID-19. Furthermore, neuropathological studies using immunostaining for aggregated α-synuclein have suggested that the PD process starts in the olfactory system or in enteric nerves and then propagates along neural pathways to additional brain regions [1.]. Indeed, hyposmia and constipation are common features of prodromal PD, and α-synuclein aggregates might contribute to their pathophysiology [1.]. Strikingly, hyposmia (and dysgeusia) are common in COVID-19, and SARS-CoV-2 can infect the gastrointestinal tract, suggesting that the virus gains direct access to brain regions relevant to PD via these routes.
Figure 1 Schematic Illustration of How Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection Might Lead to Increased Parkinson’s Disease (PD) Risk.
(A) Initial viral infection of the respiratory tract and/or the gut in patients with coronavirus disease 2019 (COVID-19) could affect the brain in three ways: through vascular damage; systemic inflammation; and direct neuroinvasion (e.g., via the olfactory system or vagal nerve), which each might act alone or in concert. Possible acute brain changes that can develop as a consequence, as well as longer term ones, are listed. Three recent case reports [3., 4., 5.] described the development of acute parkinsonism following COVID-19. These emerging findings suggest that SARS-CoV-2 infection leads to acute parkinsonism in certain cases, and raises the question of whether COVID-19 also elevates PD risk in the long term. Several of the brain changes that are commonly seen in PD (B) have also been observed following infection with SARS-CoV-2 or other related viruses, although fundamental questions remain regarding possible causal/mechanistic links.
A case of probable Parkinson's disease after SARS-CoV-2 infection
Cohen et al., The Lancet Neurology, 2020.10
“Parkinson's disease or parkinsonism have been described after infections by viruses, such as influenza A, Epstein-Barr virus, varicella zoster, hepatitis C virus, HIV, Japanese encephalitis virus, or West Nile virus. We report a patient with probable Parkinson's disease, who was diagnosed after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. …
The mechanism that led to the presumed degeneration of nigrostriatal dopaminergic nerve terminals is unclear. Perhaps a susceptible genetic makeup made our patient vulnerable to immunologically mediated mitochondrial injury and neuronal oxidative stress. Another hypothesis could be that the virus causes inflammation via microglial activation, contributing to protein aggregation and neurodegeneration. However, the short time interval between the acute infection and the parkinsonian symptoms makes this hypothesis unlikely. Other researchers have proposed the so-called multiple hit hypothesis, by which the combination of toxic stress and an inhibition of neuroprotective responses can lead to neuronal death.
Parkinson's disease is often preceded by anosmia, which is a common feature of SARS-CoV-2 infection. Immune activation in the olfactory system might eventually lead to the misfolding of α-synuclein and the development of Parkinson's disease. This mechanism is supported by post-mortem studies, showing increased levels of TNF, IL1, and IL6. Moreover, patients with Parkinson's disease had an elevated CSF antibody response to seasonal coronaviruses, compared with age-matched healthy controls.
However, we cannot exclude an interaction between other, less frequent mutations and SARS-CoV-2. The temporal association between the episode of SARS-CoV-2 infection and parkinsonian symptoms, which appeared during the acute infection, is intriguing. Before his admission to the Department of Neurology, the patient had tested negative for SARS-CoV-2 on real-time RT-PCR on two occasions; however, he was then found positive for anti-SARS-CoV-2 IgG antibodies in serum, but negative for these antibodies in CSF. Nonetheless, we cannot exclude the possibility that SARS-CoV-2 entered the CNS, particularly in view of the olfactory involvement and borderline pleocytosis.”
Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry
Romero-Sánchez et al., Neurology, 2020.06
“Results: Of 841 patients hospitalized with COVID-19 (mean age 66.4 years, 56.2% men), 57.4% developed some form of neurologic symptom. Nonspecific symptoms such as myalgias (17.2%), headache (14.1%), and dizziness (6.1%) were present mostly in the early stages of infection. Anosmia (4.9%) and dysgeusia (6.2%) tended to occur early (60% as the first clinical manifestation) and were more frequent in less severe cases. Disorders of consciousness occurred commonly (19.6%), mostly in older patients and in severe and advanced COVID-19 stages. Myopathy (3.1%), dysautonomia (2.5%), cerebrovascular diseases (1.7%), seizures (0.7%), movement disorders (0.7%), encephalitis (n = 1), Guillain-Barré syndrome (n = 1), and optic neuritis (n = 1) were also reported, but less frequent. Neurologic complications were the main cause of death in 4.1% of all deceased study participants.”
“Conclusions: Neurologic manifestations are common in hospitalized patients with COVID-19. In our series, more than half of patients presented some form of neurologic symptom. Clinicians need to maintain close neurologic surveillance for prompt recognition of these complications. The mechanisms and consequences of severe acute respiratory syndrome coronavirus type 2 neurologic involvement require further studies.”
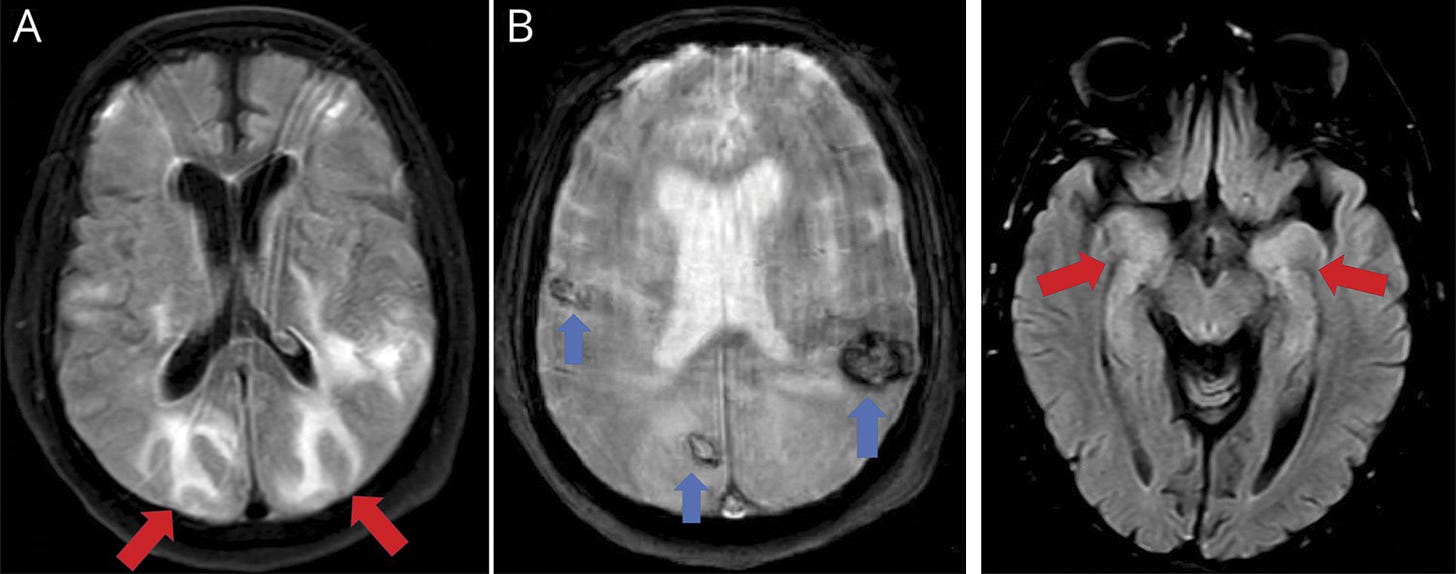
Fig. 1 (A & B). Hemorrhages and posterior reversible encephalopathy syndrome–like features
“Neuroimaging (MRI) showed bilateral subcortical hyperintense lesions with vasogenic edema in occipito-parietal lobes (A, axial fluid-attenuated inversion recovery sequence, red arrows) resembling posterior reversible encephalopathy syndrome. Gradient-echo sequences also revealed bilateral hypointense lesions compatible with several hemorrhages (B, axial T2 gradient echo sequence, blue arrows). MRI excluded other possibilities such as cerebral venous sinus thrombosis.”
Fig. 2 (right). Bitemporal lobe involvement compatible with encephalitis
“MRI axial fluid-attenuated inversion recovery sequence showed bilateral hyperintensity within both temporo-mesial lobes (red arrows), compatible with encephalitis. CSF was normal, including real-time reverse transcription–polymerase chain reaction for RNA of SARS-CoV-2.”