Bridle et al., CCCA, 2022.01
“Our critique of the Thomas et al. publication revealed multiple concerns regarding author claims of BNT162b2 safety and efficacy as well as a high number of direct conflicts of interest in the publication authors. These, coupled with multiple reports indicating that vaccine efficacy wanes within months of administration (20-23), reduced effectiveness of BNT162b2 with respect to emerging variants (24-26), record rates of serious adverse events (122,833) and deaths (17,128) reported in the US passive Vaccine Adverse Event Reporting System, VAERS by October 16, 2021, and problems with data integrity in the conduct of this trial reported recently by Thacker (1) in the British Medical Journal, raise further concerns regarding both the efficacy and safety of this agent. We did not find sufficient evidence to support use of these agents in the healthy adults studied or in specific unstudied demographics that are being mandated to comply with vaccination including the naturally immune, the frail elderly, those with multiple co-morbidities, the immunocompromised, and pregnant women. It also calls into question use in adolescents and children given that companion trials conducted in those populations suffered from similar design flaws, including underpowered in participant numbers and that recommendations for use were based on minimal safety follow up.”
Pfizer 6-month data report: analysis of trial flaws in design and execution, 2021.12
Covid-19: Researcher blows the whistle on data integrity issues in
FDA Buries Data on Seriously Injured Child in Pfizer’s C-19 Clinical Trial, 📹
Multisystem Inflammatory-like Syndrome in a Child Following COVID-19 mRNA Vaccination
Poussaint et al., Vaccines, 2021.12
“A 12-year-old male was presented to the hospital with acute encephalopathy, headache, vomiting, diarrhea, and elevated troponin after recent COVID-19 vaccination. Two days prior to admission and before symptom onset, he received the second dose of the Pfizer-BioNTech COVID-19 vaccine. Symptoms developed within 24 h with worsening neurologic symptoms, necessitating admission to the pediatric intensive care unit. Brain magnetic resonance imaging within 16 h of admission revealed a cytotoxic splenial lesion of the corpus callosum (CLOCC). Nineteen days prior to admission, he developed erythema migrans, and completed an amoxicillin treatment course for clinical Lyme disease. However, Lyme antibody titers were negative on admission and nine days later, making active Lyme disease an unlikely explanation for his presentation to hospital. An extensive workup for other etiologies on cerebrospinal fluid and blood samples was negative, including infectious and autoimmune causes and known immune deficiencies. Three weeks after hospital discharge, all of his symptoms had dissipated, and he had a normal neurologic exam. Our report highlights a potential role of mRNA vaccine-induced immunity leading to MIS-C-like symptoms with cardiac involvement and a CLOCC in a recently vaccinated child and the complexity of establishing a causal association with vaccination. The child recovered without receipt of immune modulatory treatment.”
Figure 2. Cytotoxic lesion of the corpus callosum. Axial T2 image (A) shows focus of T2 prolongation representing cytotoxic edema in the splenium of corpus callosum (arrow) with reduced diffusivity on trace diffusion image (B) (arrow) and apparent diffusion coefficient map (C).
The Pfizer mRNA vaccine: pharmacokinetics and toxicity
Palmer and Bhakdi, 2021.07.23
“We summarize the findings of an animal study which Pfizer submitted to the Japanese health authorities in 2020, and which pertained to the distribution and elimination of a model mRNA vaccine. We show that this study clearly presaged grave risks of blood clotting and other adverse effects. The failure to monitor and assess these risks in the subsequent clinical trials, and the grossly negligent review process in conjunction with the emergency use authorizations, have predictably resulted in an unprecedented medical disaster.”
Summary
Pfizer’s animal data clearly presaged the following risks and dangers:
blood clotting shortly after vaccination, potentially leading to heart attacks, stroke, and venous thrombosis
grave harm to female fertility
grave harm to breastfed infants
cumulative toxicity after multiple injections
“Of particularly grave concern is the very slow elimination of the toxic cationic lipids. In persons repeatedly injected with mRNA vaccines containing these lipids— be they directed against COVID, or any other pathogen or disease—this would result in cumulative toxicity. There is a real possibility that cationic lipids will accumulate in the ovaries. The implied grave risk to female fertility demands the most urgent attention of the public and of the health authorities.”
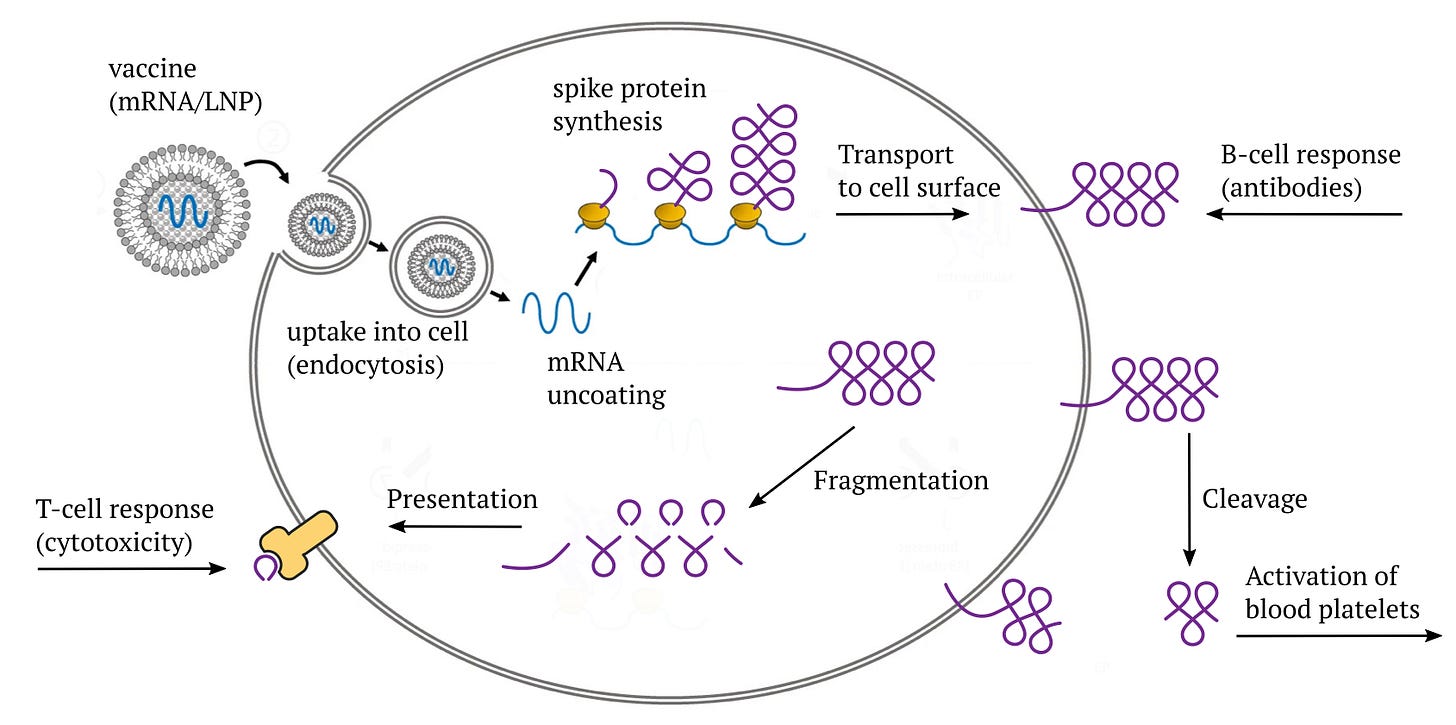
Fig. 1. mRNA COVID vaccines
Dr. Malone discusses the Pfizer Japanese study (44 min ⟶)
The BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses
Föhse et al., medRxiv, 2021.05
“The mRNA-based BNT162b2 vaccine from Pfizer/BioNTech was the first registered COVID-19 vaccine and has been shown to be up to 95% effective in preventing SARS-CoV-2 infections. Little is known about the broad effects of the new class of mRNA vaccines, especially whether they have combined effects on innate and adaptive immune responses. Here we confirmed that BNT162b2 vaccination of healthy individuals induced effective humoral and cellular immunity against several SARS-CoV-2 variants. Interestingly, however, the BNT162b2 vaccine also modulated the production of inflammatory cytokines by innate immune cells upon stimulation with both specific (SARS-CoV-2) and non-specific (viral, fungal and bacterial) stimuli. The response of innate immune cells to TLR4 and TLR7/8 ligands was lower after BNT162b2 vaccination, while fungi-induced cytokine responses were stronger. In conclusion, the mRNA BNT162b2 vaccine induces complex functional reprogramming of innate immune responses, which should be considered in the development and use of this new class of vaccines.”
Havla et al., Journal of Neurology, 2021.05
“A 28-year-old woman developed the first clinical manifestation of relapsing MS after vaccination with the Pfizer-BioNTech COVID-19 vaccine (BNT162b2, Comirnaty©, BioNTech/Pfizer). Six days after the initially well-tolerated first immunization, she began to develop left abdominal neuropathic pain, sensory impairment below the T6 level, with hypoesthesia of right abdominal wall and genital regions, and left leg paresis. Magnetic resonance imaging (MRI) of the spinal cord on day 18 after vaccination showed a contrast-enhancing lesion at the T6 level, suggestive of myelitis, and cerebral MRI revealed multiple (> 20), partially confluent lesions with spatial dissemination but no Gadolinium enhancement. On cerebrospinal fluid (CSF) analysis mild pleocytosis (7 cells/µl) and oligoclonal bands were found. In line with a positive vaccine reaction, SARS-CoV-2 S antibodies (abs, IgG; Roche) were detected in serum (50.8 U/ml, 37 days after vaccination). SARS-CoV-2 infection was excluded on the basis of a negative PCR and absence of antibodies against the SARS-CoV-2 N protein (abs; Roche).”
Fig. 2. Cranial MRI performed one week after spinal MRI (Fig. 1). 3D FLAIR with 1 mm slice thickness and reconstruction in three planes. a The sagittal image shows a lesion in the splenium of the corpus callosum. b Axial image shows a periventricular lesion with triangular configuration. c Coronal image depicts a juxtacortical lesion involving the U-fibers. d Axial image shows involvement of the cerebellum. Overall, the MRI showed more than 20 specific lesions larger than 3 mm at periventricular, cortical/juxtacortical, or infratentorial locations without contrast enhancement