Perez, Moret-Chalmin, and Montagnier, ResearchGate preprint, 2022.02
“We highlight the presence of a Prion region in the different Spike proteins of the original SARS-CoV2 virus as well as of all its successive variants but also of all the “vaccines” built on this same sequence of the Spike SARS-CoV2 from Wuhan.
Paradoxically, with a density of mutations 8 times greater than that of the rest of the spike, the possible harmfulness of this Prion region disappears completely in the Omicron variant. We analyze and explain the causes of this disappearance of the Prion region of the Spike of Omicron. At the same time, we are analyzing the concomitance of cases, which occurred in various European countries, between the first doses of Pfizer or Moderna mRNA vaccine and the sudden and rapid onset of the first symptoms of Creutzfeldt- Jakob disease, which usually requires several years before observing its first symptoms.
We are studying 16 Creutzfeld Jakob Diseases, in 2021, from an anamnestic point of view, centered on the chronological aspect of the evolution of this new prion disease, without being able to have an explanation of the etiopathogenic aspect of this new entity. We subsequently recall the usual history of this dreadfull subacute disease, and compare it with this new, extremely acute, prion disease, following closely vaccinations. In a few weeks, more 40 cases of almost spontaneous emergence of Creutzfeldt-Jakob disease have appeared in France and Europe very soon after the injection of the first or second dose of Pfizer, Moderna or AstraZeneka vaccines. We report here 16 cases from France, Belgium, Switzerland and Israel. We can place special emphasis on the remarkably very similar timing of the clinical semeiology. Indeed, of the 16 cases studied here, the first symptoms appeared on average 11.06 days after the injection (with a dispersion ranging from a minimum of 1 day to a maximum of 30 days.
We suggest the reasons for thinking that we are dealing here with an entirely new form of Creutzfeldt-Jakob disease.”
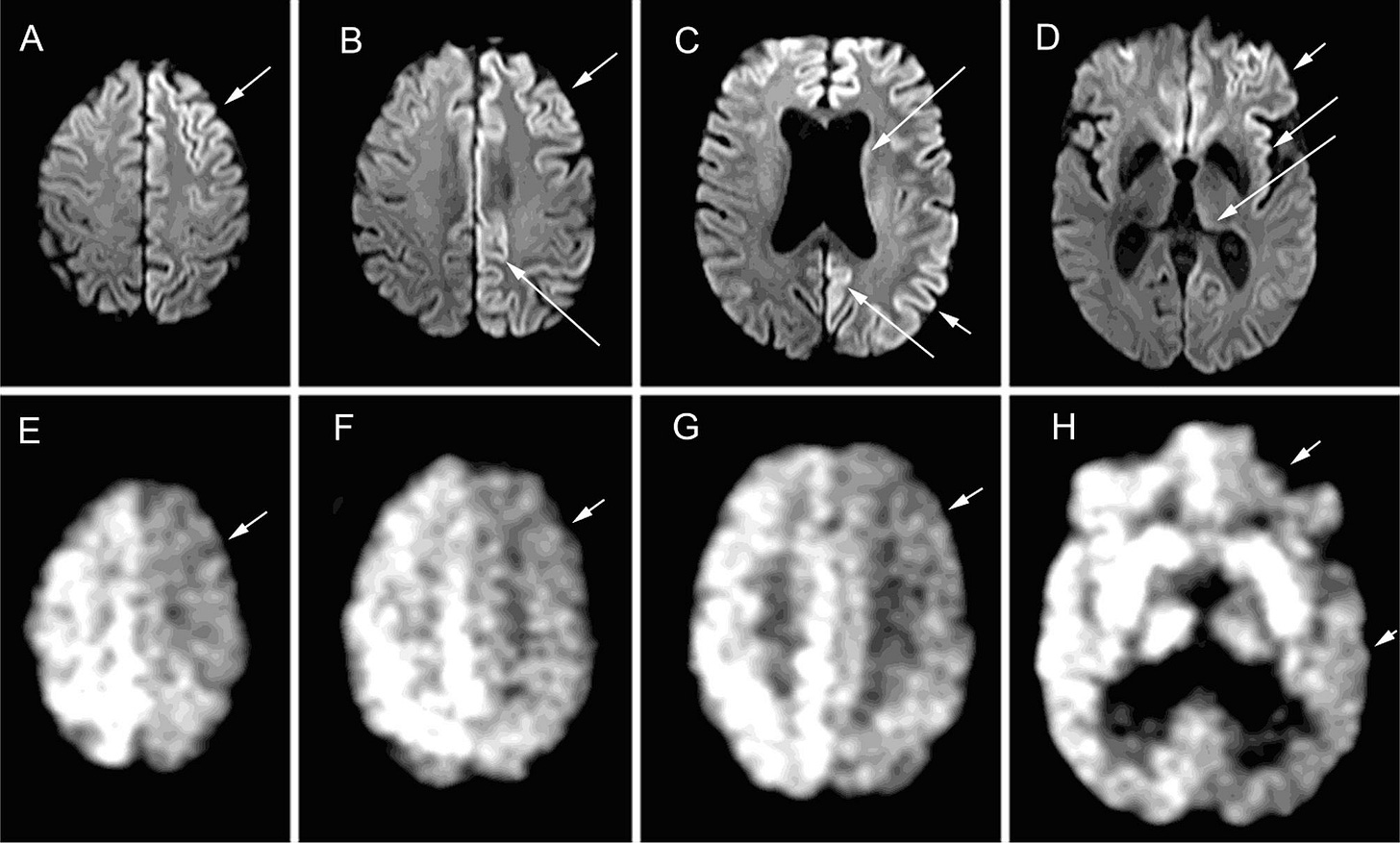
Figure 31 – The case of M.D.: MRI, PET and EEG (D. M) -Brain MRI (Diffusion Weighted Imaging) and (Fluid-Attenuated Inversion Recovery : FLAIR) and (T2) : abnormalities of parietal lobes predominantly on the left side and of cingulate gyrus. -FDG-PET : hypometabolism of right hemisphere predominantly in the right frontal and parietal lobes. -EEG : 6Hz background activity and 6 seconds of 1Hz triphasic periodic spikes in the right hemisphere.
The blue rectangle in the EEG is a typical proof of CREUTZFELDT-JAKOB disease (6 seconds of 1 Hertz triphasic periodic spikes).
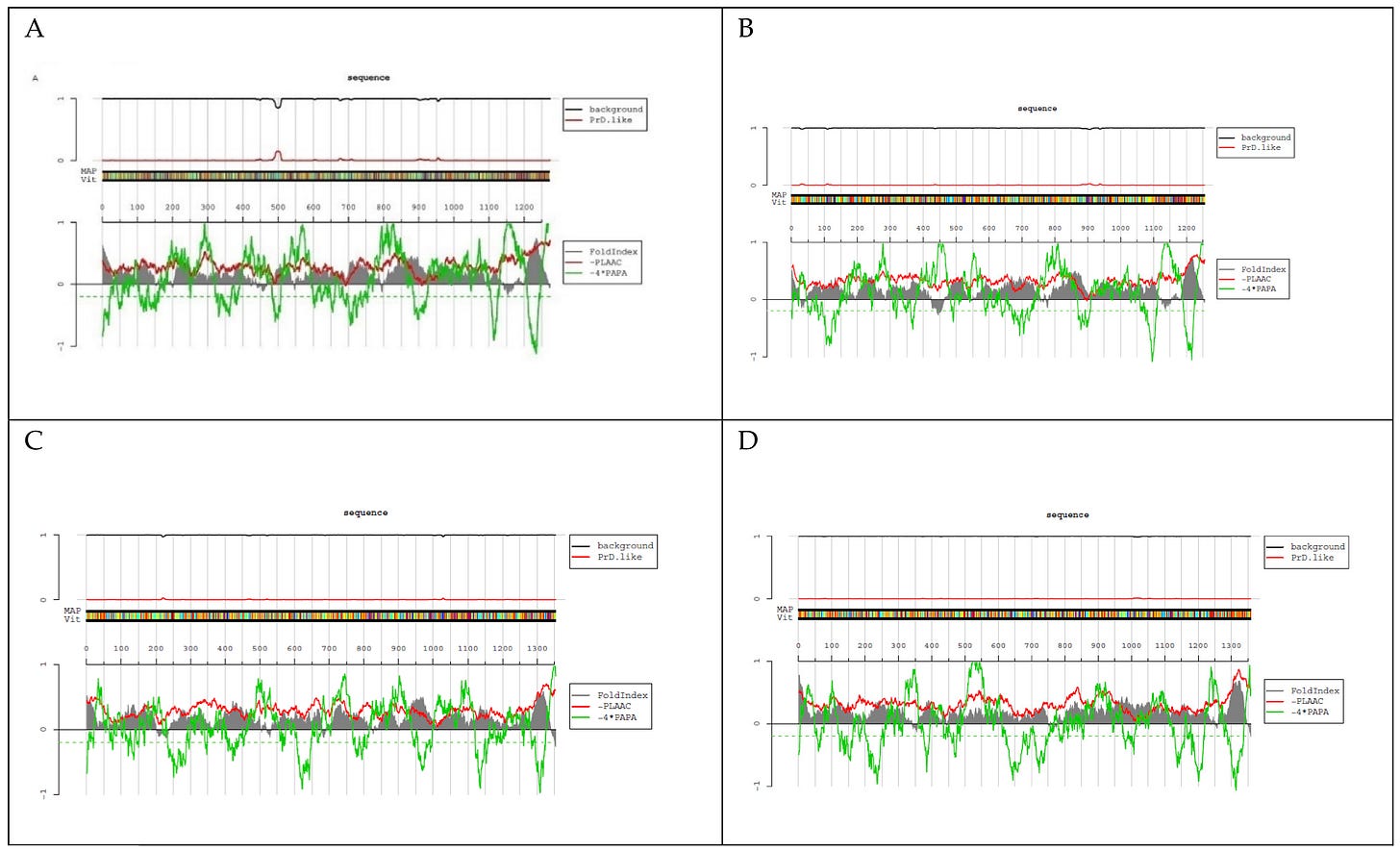
Figure 24 – PLAAC software demonstrates the presence of the Prion region around amino acids 500 of the spike of both vaccine Moderna.
Table 2 – Presence of the Prion region in ALL historical SARS-CoV2 Spikes excepted in Bat RaTG13.
Conclusions
“Etiopathogenic hypothesis remains mysterious and deserves far more further investigations. We only discuss a new type of Creutzfeld Jakob because of the acute onset and the fatal very rapid issue as well as the immediate triggering effect of mRNA based immunotherapy. Increase in frequency of CJD or spongiform encephalopathy or prion diseases is still to confirm, worldwide. The first results, in France, Belgium, Switzerland and Israel suggest high increase. Finally, we will retain 3 major results of this study:
–First, we demonstrate the existence of a Prion region in all the Spikes of the original SARS-CoV2 strain from Wuhan, of all the variants and of all the "vaccines" since they were all constructed from this original spike from Wuhan.
–Second, we demonstrate that this Prion region has totally disappeared in the latest Omicron variant. This can be explained by the philogenic tree of the SARS-CoV2 viruses, of which the Omicron is the result of one of the very first branches, then it would have evolved quietly in sleep in South Africa, to finally emerge in November 2021. in a form that was to become dominant.
–Finally, and this is the third remarkable result, if the presence of this Prion region in all COVID-19 vaccines constituted "a necessary but not sufficient reason" for the emergence of a possible Prion disease, we bring here the formal evidence of this new form of CJD soon after injection. Indeed, of the 16 cases studied here, the first symptoms appeared on average 11.06 days after the injection (with a dispersion ranging from a minimum of 1 day to a maximum of 30 days.”
Tetz and Tetz, Microorganisms, 2022.01
“Prions are proteins that possess a unique conformational conversion, with the ability to rapidly shift between multiple conformations due to residue hydrophobicity and net sequence charge, and viral prion-like proteins are known as potential regulators of viral infections. However, the prion-like domains (PrD) in the SARS-CoV-2 proteome have not been analyzed. In this in silico study, using the PLAAC algorithm, we identified the presence of prion-like domains in the SARS-CoV-2 spike protein. Compared with other viruses, a striking difference was observed in the distribution of prion-like domains in the spike protein since SARS-CoV-2 is the only coronavirus with a prion-like domain found in the receptor-binding domain of the S1 region of the spike protein. The presence and unique distribution of prion-like domains in the SARS-CoV-2 receptor-binding domains of the spike protein are particularly interesting since although the SARS-CoV-2 and SARS-CoV S proteins share the same host cell receptor, angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 demonstrates a 10- to 20-fold higher affinity for ACE2. We identified prion-like domains in the α1 helix of the ACE2 receptor that interact with the viral receptor-binding domain of SARS-CoV-2. Finally, we found substantial differences in the prion-like domain of the S1 region of the spike protein across emerging variants including Omicron (B.1.1.529). Taken together, the present findings indicate that the identified PrDs in the SARS-CoV-2 receptor-binding domain (RBD) and ACE2 region that interact with RBD play important functional roles in viral adhesion and entry.”
Fig. 1 Analysis and comparison of mutations in the RBD of SARS-CoV-2 and SARS-CoV. The RBD of the SARS-CoV-2 (NCBI reference sequence: YP_009724390.1) spike protein was aligned with the closest related human βCoV, SARS-CoV (NCBI reference sequence: AYV99817.1). The PrDs of SARS-CoV-2 are red. Different residues are denoted by * beneath the consensus position. The amino acids asparagine (Q) and glutamine (N) in the PrDs of the SARS-CoV-2 RBD that differ from the amino acids in the SARS-CoV RBD are denoted by a red ** beneath the consensus position. Amino acids of the SARS-CoV-2 RBD that bind to ACE2 are marked with red boxes. RBD—receptor-binding domain.
Fig. 3 Heatmap showing PrD within the S protein in SARS-CoV-2 variants. The correlation between the LLR scores of the identified PrDs in the S protein across different SARS-CoV-2 variants is presented. Mean LLC scores of S protein are denoted using a color scale, ranging from white (minimum) to saturated red (maximum). Higher LLC scores indicate a higher possibility that the analyzed protein is a prion.
Figure S1. The LLR value of the S protein from (A) SARS-CoV-2 (LLR 4.856), (B) SARS-CoV (LLR 4.426), (C) MERS- CoV (LLR 4.49) and (D) HcoV-OC43 (LLR 2.828).
DIY PLAAC analysis
PLAAC: Prion-Like Amino Acid Composition (Lancaster et al., 2014)
SARS-CoV-2 isolate Wuhan-Hu-1, spike glycoprotein (Wu et al., 2020.02)
SARS-CoV-2 surface glycoprotein: Protein ID=YP_009724390.1
GeneID: 43740568
MFVFLVLLPLVSSQCVNLTTRTQLPPAYTNSFTRGVYYPDKVFR SSVLHSTQDLFLPFFSNVTWFHAIHVSGTNGTKRFDNPVLPFNDGVYFASTEKSNIIR GWIFGTTLDSKTQSLLIVNNATNVVIKVCEFQFCNDPFLGVYYHKNNKSWMESEFRVY SSANNCTFEYVSQPFLMDLEGKQGNFKNLREFVFKNIDGYFKIYSKHTPINLVRDLPQ GFSALEPLVDLPIGINITRFQTLLALHRSYLTPGDSSSGWTAGAAAYYVGYLQPRTFL LKYNENGTITDAVDCALDPLSETKCTLKSFTVEKGIYQTSNFRVQPTESIVRFPNITN LCPFGEVFNATRFASVYAWNRKRISNCVADYSVLYNSASFSTFKCYGVSPTKLNDLCF TNVYADSFVIRGDEVRQIAPGQTGKIADYNYKLPDDFTGCVIAWNSNNLDSKVGGNYN YLYRLFRKSNLKPFERDISTEIYQAGSTPCNGVEGFNCYFPLQSYGFQPTNGVGYQPY RVVVLSFELLHAPATVCGPKKSTNLVKNKCVNFNFNGLTGTGVLTESNKKFLPFQQFG RDIADTTDAVRDPQTLEILDITPCSFGGVSVITPGTNTSNQVAVLYQDVNCTEVPVAI HADQLTPTWRVYSTGSNVFQTRAGCLIGAEHVNNSYECDIPIGAGICASYQTQTNSPR RARSVASQSIIAYTMSLGAENSVAYSNNSIAIPTNFTISVTTEILPVSMTKTSVDCTM YICGDSTECSNLLLQYGSFCTQLNRALTGIAVEQDKNTQEVFAQVKQIYKTPPIKDFG GFNFSQILPDPSKPSKRSFIEDLLFNKVTLADAGFIKQYGDCLGDIAARDLICAQKFN GLTVLPPLLTDEMIAQYTSALLAGTITSGWTFGAGAALQIPFAMQMAYRFNGIGVTQN VLYENQKLIANQFNSAIGKIQDSLSSTASALGKLQDVVNQNAQALNTLVKQLSSNFGA ISSVLNDILSRLDKVEAEVQIDRLITGRLQSLQTYVTQQLIRAAEIRASANLAATKMS ECVLGQSKRVDFCGKGYHLMSFPQSAPHGVVFLHVTYVPAQEKNFTTAPAICHDGKAH FPREGVFVSNGTHWFVTQRNFYEPQIITTDNTFVSGNCDVVIGIVNNTVYDPLQPELD SFKEELDKYFKNHTSPDVDLGDISGINASVVNIQKEIDRLNEVAKNLNESLIDLQELG KYEQYIKWPWYIWLGFIAGLIAIVMVTIMLCCMTSCCSCLKGCCSCGSCCKFDEDDSE PVLKGVKLHYT
Carossino et al., bioRxiv, 2021.09
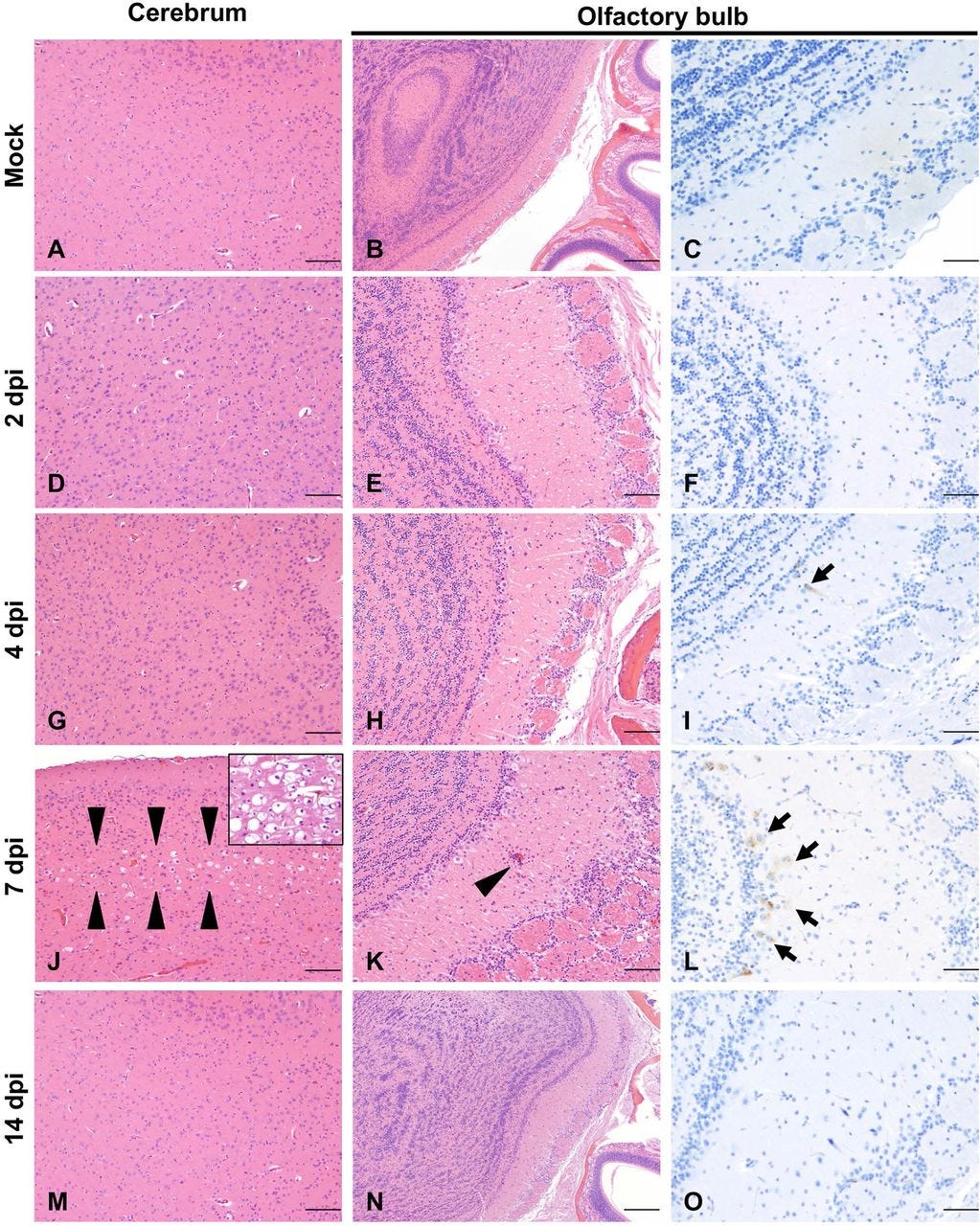
"Histologic findings included moderate to marked neuronal spongiosis, multifocal shrunken, angular, hypereosinophilic and pyknotic neuronal bodies with loss of Nissl substance/chromatolysis (neuronal degeneration and necrosis) and occasionally delimited by multiple glial cells (satellitosis). In the olfactory bulb, delicate lymphocytic perivascular cuffing and a general increase in the number of reactive glial cells (gliosis) were evident in the neuroparenchyma neighboring areas of neuronal degeneration and necrosis."
Fig. 7 Temporal neuronal damage in K18-hACE2 mice following intranasal inoculation with SARS-CoV-2.
Histologic changes in the cerebrum (A, D, G, J, M) or olfactory bulb (B, E, H, K, N) in non-infected or infected K18-hACE2 mice. SARS-CoV-2 Spike protein was also probed in the olfactory bulb of non-infected and infected K18-hACE2 mice by IHC (C, F, I, L, O). SARS-CoV-2 protein (brown) was evident as early as 4 dpi (I, arrow), but no histologic changes were noted until 7 dpi (J-K). At that time point, mild (J, arrowheads) to marked (J, inset) spongiosis with neuronal degeneration and necrosis involving multiple areas within the cerebral cortex and elsewhere were observed. Similar changes were evident in the olfactory bulb, with occasional perivascular cuffs/gliosis (K, arrowhead) and abundant viral protein (L, arrows). No histologic alterations or viral protein were detected in survivor mice euthanized at 14 dpi (M-O). H&E and DAB (viral protein), 100X (A, B, D, E, G, H, J, K, M, N; bar = 200 μm) and 200X (C, F, I, L, O; bar = 100 μm) total magnification.
SARS-CoV-2 causes brain inflammation and induces Lewy body formation in macaques
Philippens et al., bioRxiv, 2021.05
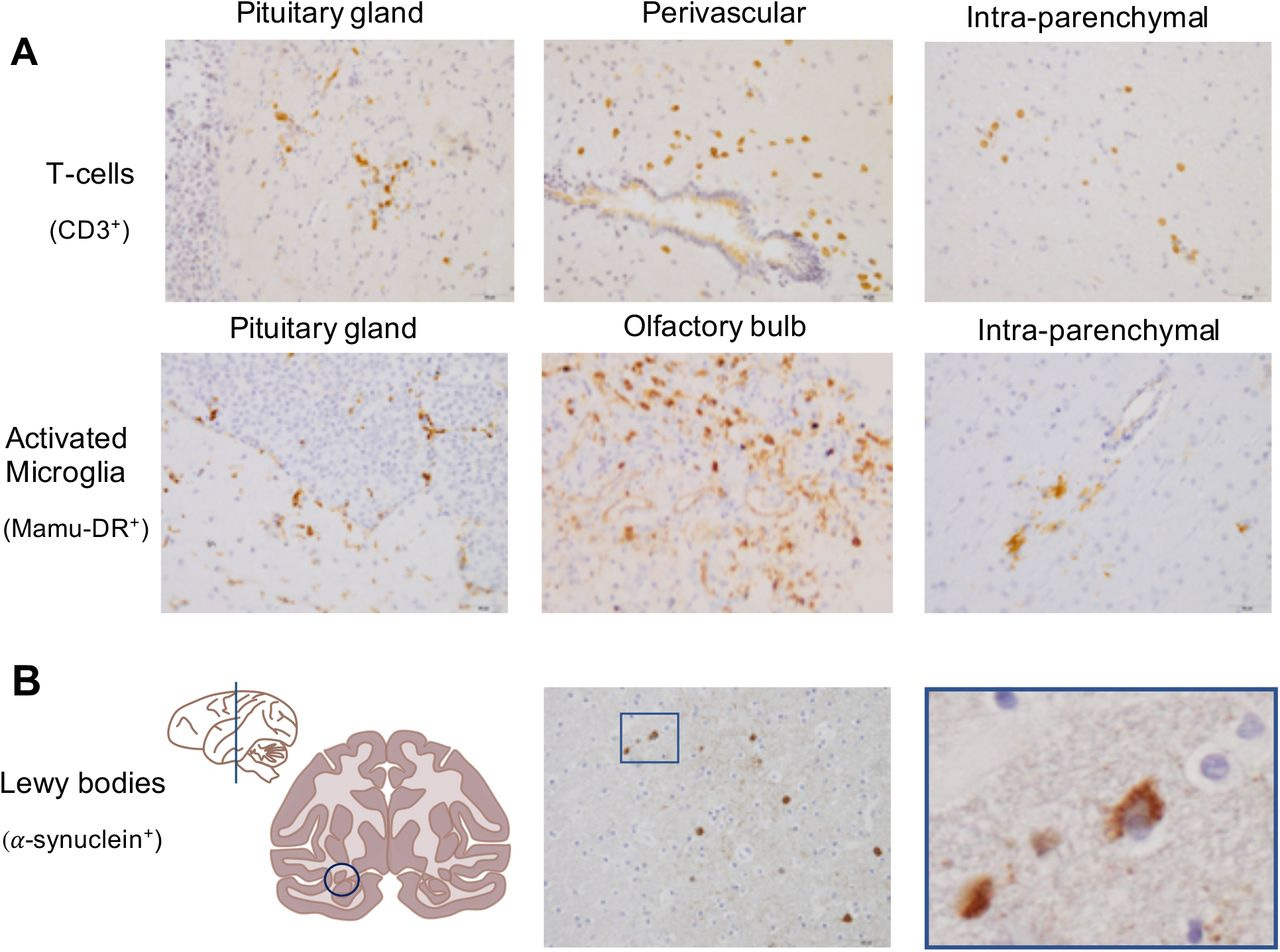
"Post-mortem analysis demonstrated infiltration of T-cells and activated microglia in the brain, and viral RNA was detected in brain tissues from one animal. We observed Lewy bodies in brains of all rhesus macaques. These data emphasize the virus’ capability to induce neuropathology in this nonhuman primate model for SARS-CoV-2 infection. As in humans, Lewy body formation is an indication for the development of Parkinson’s disease, this data represents a warning for potential long-term neurological effects after SARS-CoV-2 infection."
Fig. 3 SARS-CoV-2 causes brain inflammation and Lewy body formation in brains of macaques.
Immunohistochemical stainings of macaque brain tissues. (A) top panel: T-cells (CD3+) in the pituitary gland (left), perivascular (middle) and in the brain parenchyma (right) from a rhesus macaque (20x). (A) middle panel: activated microglia (Mamu-DR+) in the pituitary gland (left), olfactory bulb (middle) and in the brain parenchyma (right) from a cynomolgus macaque (20x). (B) bottom panel: Lewy bodies (α-synuclein +) were found in the ventral midbrain region next to the caudate nucleus indicated with a circle (left) in the coronal section of one hemisphere (anterior-posterior 0), presence of Lewy bodies in a SARS-CoV-2-infected rhesus macaque (20x) (middle), a magnified image (40x) of the square in the left image (right).
Seneff and Nigh, Int. Journal of Vaccine Theory, Practice, & Research, 2021.05
“Operation Warp Speed brought to market in the United States two mRNA vaccines, produced by Pfizer and Moderna. Interim data suggested high efficacy for both of these vaccines, which helped legitimize Emergency Use Authorization (EUA) by the FDA. However, the exceptionally rapid movement of these vaccines through controlled trials and into mass deployment raises multiple safety concerns. In this review we first describe the technology underlying these vaccines in detail. We then review both components of and the intended biological response to these vaccines, including production of the spike protein itself, and their potential relationship to a wide range of both acute and long-term induced pathologies, such as blood disorders, neurodegenerative diseases and autoimmune diseases. Among these potential induced pathologies, we discuss the relevance of prion-protein-related amino acid sequences within the spike protein. We also present a brief review of studies supporting the potential for spike protein “shedding”, transmission of the protein from a vaccinated to an unvaccinated person, resulting in symptoms induced in the latter. We finish by addressing a common point of debate, namely, whether or not these vaccines could modify the DNA of those receiving the vaccination. While there are no studies demonstrating definitively that this is happening, we provide a plausible scenario, supported by previously established pathways for transformation and transport of genetic material, whereby injected mRNA could ultimately be incorporated into germ cell DNA for transgenerational transmission. We conclude with our recommendations regarding surveillance that will help to clarify the long-term effects of these experimental drugs and allow us to better assess the true risk/benefit ratio of these novel technologies.”
Figure 2: A simple model for a process by which the spike protein produced through the mRNA vaccines could induce a pathological response distinct from the desirable induction of antibodies to suppress viral entry. Redrawn with permission from Suzuki & Gychka, 2021.
Fig 2. (Suzuki and Gychka, 2021) Biological functions of ACE2. In physiological situations, ACE2 functions as a carboxypeptidase enzyme that catalyzes the hydrolysis of angiotensin II (Ang II) into Ang(1–7) by cleaving off a phenylalanine (Phe). In the presence of the spike protein, this enzyme becomes a membrane receptor for cell signaling that uses the spike protein as a ligand for its activation.
Idrees and Kumar, Biochemical & Biophysical Research Communications, 2021.03
“The post-infection of COVID-19 includes a myriad of neurologic symptoms including neurodegeneration. Protein aggregation in brain can be considered as one of the important reasons behind the neurodegeneration. SARS-CoV-2 Spike S1 protein receptor binding domain (SARS-CoV-2 S1 RBD) binds to heparin and heparin binding proteins. Moreover, heparin binding accelerates the aggregation of the pathological amyloid proteins present in the brain. In this paper, we have shown that the SARS-CoV-2 S1 RBD binds to a number of aggregation-prone, heparin binding proteins including Aβ, α-synuclein, tau, prion, and TDP-43 RRM. These interactions suggests that the heparin-binding site on the S1 protein might assist the binding of amyloid proteins to the viral surface and thus could initiate aggregation of these proteins and finally leads to neurodegeneration in brain. The results will help us to prevent future outcomes of neurodegeneration by targeting this binding and aggregation process.”
Fig. 2. SARS-CoV-2 spike protein S1 RBD domain interactions to Heparin. (A) Docking model of the interaction of heparin (grey) to heparin-binding domains of spike protein, S1 (green). (B) The Ligplot diagram showing the detailed molecular interactions between S1 and heparin as observed by PDBsum.
Fig. from “COVID-19 Vaccines & neurodegenerative disease”, S. Seneff, PhD, 2022.01
Prion Diseases: A Unique Transmissible Agent or a Model for Neurodegenerative Diseases?
Ritchie and Barria, Biomolecules, 2021.02
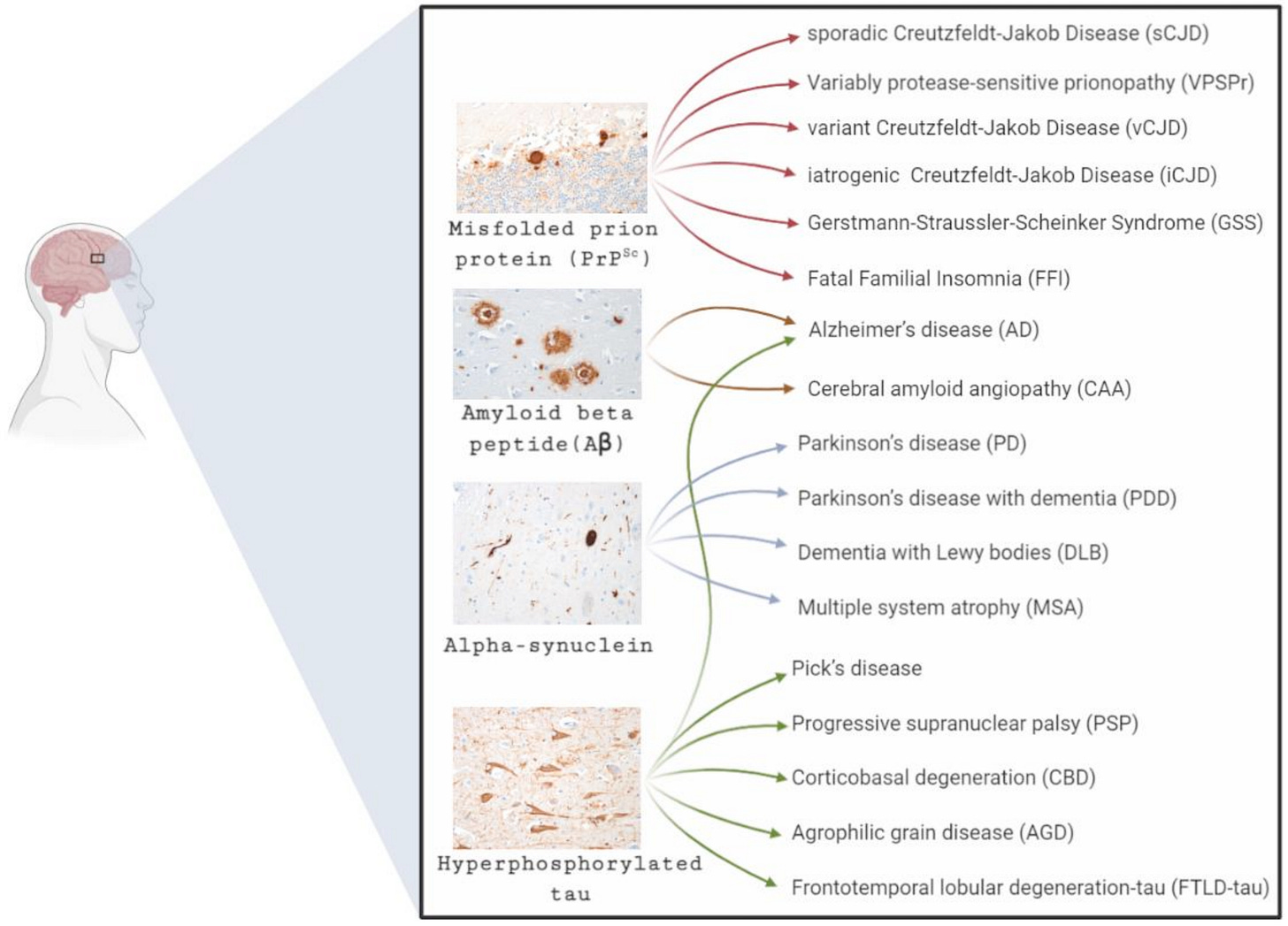
“The accumulation and propagation in the brain of misfolded proteins is a pathological hallmark shared by many neurodegenerative diseases such as Alzheimer’s disease (Aβ and tau), Parkinson’s disease (α-synuclein), and prion disease (prion protein). Currently, there is no epidemiological evidence to suggest that neurodegenerative disorders are infectious, apart from prion diseases. However, there is an increasing body of evidence from experimental models to suggest that other pathogenic proteins such as Aβ and tau can propagate in vivo and in vitro in a prion-like mechanism, inducing the formation of misfolded protein aggregates such as amyloid plaques and neurofibrillary tangles. Such similarities have raised concerns that misfolded proteins, other than the prion protein, could potentially transmit from person-to-person as rare events after lengthy incubation periods. Such concerns have been heightened following a number of recent reports of the possible inadvertent transmission of Aβ pathology via medical and surgical procedures. This review will provide a historical perspective on the unique transmissible nature of prion diseases, examining their impact on public health and the ongoing concerns raised by this rare group of disorders. Additionally, this review will provide an insight into current evidence supporting the potential transmissibility of other pathogenic proteins associated with more common neurodegenerative disorders and the potential implications for public health.”
Figure 1. Common neurodegenerative diseases and the predominant protein accumulation in the brain. The most common misfolded proteins associated with neurodegenerative diseases are amyloid-beta (Aβ), tau, and alpha-synuclein (α-synuclein). These protein aggregates are represented in the four different micrographs. In some instances, the neurodegenerative conditions are associated with the accumulation in the brain of a single major protein type; this is best described in prion diseases. However, in other disorders such as Alzheimer’s disease, multiple major protein forms are described. With a number of conditions characterised by the same protein aggregation, differential diagnosis is often dependent on the clinical phenotype.
Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration?
Young et al., Brain, Behavior, and Immunity, 2020.07
“We describe a man whose first manifestations of Creutzfeldt-Jakob disease occurred in tandem with symptomatic onset of coronavirus disease 2019 (COVID-19). Drawing from recent data on prion disease pathogenesis and immune responses to SARS-CoV-2, we hypothesize that the cascade of systemic inflammatory mediators in response to the virus accelerated the pathogenesis of our patient’s prion disease. This hypothesis introduces the potential relationship between immune responses to the novel coronavirus and the hastening of preclinical or manifest neurodegenerative disorders. The global prevalence of both COVID-19 and neurodegenerative disorders adds urgency to the study of this potential relationship.”
Fig. 2. A – D: Brain MRI findings in this case. Diffusion weighted images from A, superior to D, inferior brain regions showing asymmetric restricted diffusion (bright signal, arrows) in the cortical ribbon more prominently in the left hemisphere in prefrontal, posterior parietal and retrosplenial cortices, as well as the insula and cingulate gyrus, with faint signal abnormality also in the body of the caudate nucleus (in C) and pulvinar of thalamus (in D). E-F: 18-FDG PET findings in this case, showing hypometabolism in areas of restricted diffusion.
Butnaru and Chapman, Autoimmunity Reviews, 2019.03
“The central nervous system (CNS) in neurodegenerative diseases is a battlefield in which microglia fight a highly atypical battle. During the inflammatory process microglia themselves become dysfunctional and even with all the available immune arsenal including cytokine or/and antibody production, the battle is eventually lost. A closer look into the picture will reveal the fact that this is mainly due to the atypical characteristics of the infectious agent. The supramolecular assemblies of misfolded proteins carry unique features not encountered in any of the common pathogens. Through misfolding, proteins undergo conformational changes which make them become immunogenic, neurotoxic and highly infective. The immunogenicity appears to be triggered by the exposure of previously hidden hydrophobic portions in proteins which act as damage-associated molecular patters (DAMPs) for the immune system. The neurotoxicity and infectivity are promoted by the small oligomeric forms of misfolded proteins/peptides. Oligomers adopt conformations such as tubular-like, beta-barrel-like, etc., that penetrate cell membranes through their hydrophobic surfaces, thus destabilizing ionic homeostasis. At the same time, oligomers act as a seed for protein misfolding through a prion/prion-like mechanism. Here, we propose the hypothesis that oligomers have catalytic surfaces and exercise their capacity to infect native proteins through specific characteristics such as hydrophobic, electrostatic and π-π stacking interactions as well as the specific surface area (SSA), surface curvature and surface chemistry of their nanoscale supramolecular assemblies. All these are the key elements for prion/prion-like mechanism of self-replication and disease spreading within the CNS. Thus, understanding the mechanism of prion's templating activity may help us in the prevention and development of novel therapeutic strategies for neurodegenerative diseases.”
Fig. from: “COVID-19 Vaccines & Neurodegenerative Disease”, Dr. S. Seneff, 2022 📹
Protein misfolding in neurodegenerative diseases: implications and strategies
Sweeney et al., Translational Neurodegeneration, 2017.03
Roles of misfolded proteins and aggregates in proteinopathies
Misfolded proteins exist in cells together with unfolded, intermediately folded, and correctly folded species. In healthy cells, misfolded proteins are either degraded or refolded correctly by chaperone proteins that are involved in protein folding and trafficking as well as intermediate stabilization [1]. Indeed, it is now believed that many, if not all, proteins can form amyloid fibrils under appropriate biochemical conditions. However, many disease-associated amyloidogenic proteins have extensive regions of intrinsic disorder in their free soluble forms [4] and have specific, often short, internal amino acid sequences that are necessary and sufficient to support aggregation. These same motifs can be found in other non-disease proteins, and when liberated from rest of protein these fragments will aggregate into cytotoxic amyloid fibrils.
Once formed, higher order amyloid aggregates are highly resistant to degradation. Proteasomes can degrade only single chain polypeptides, and also require the proteins to be partially or fully (in the case of proteasomes) unfolded. In addition, the amyloid state is extremely stable thermodynamically, because of the extensive contacts made between the protein chains of the polymer. The thermodynamic stability of amyloid aggregates also contributes to their ability to convert native proteins into amyloid forms (i.e., to seed prion-like propagation).
Mechanisms of misfolded protein toxicity
In the long term, all neurodegenerative disease proteins produce synaptic dysfunction and loss and, ultimately, neuronal cell death. The precise upstream mechanisms by which different misfolded disease proteins cause neurotoxicity are still unclear, and appear to differ depending on the protein species involved. Misfolded disease proteins appear to act primarily by toxic gain-of-function and/or dominant-negative effects, although loss-of-function effects have also been observed. Direct, acute effects of misfolded proteins on neuronal function have been observed after treating neurons with purified oligomers or transfecting them with expression vectors. To give just a few examples, amyloid-beta, tau, and alpha-synuclein all interfere with synaptic signaling; mutant tau disrupts microtubule function and neuronal transport mechanisms; and alpha-synuclein disrupts mitochondrial protein import. In addition, larger aggregates of misfolded proteins may exert toxic effects by binding to and sequestering other cytosolic proteins. For example, proteomic studies of artificial proteins designed to form amyloid-like fibrils showed that the toxicity of these proteins correlated with the ability of their aggregates to engage in aberrant protein interactions and disrupt the cytosolic stress response. …
In addition to synaptic dysfunction, other cellular changes common to the major neurodegenerative diseases include calcium signaling abnormalities, mitochondrial dysfunction, oxidative stress, and neuroinflammation. These symptoms of cellular distress often occur early in the disease process, and are believed to be a cause as well as a consequence of neurodegeneration. That is, the relationship between the accumulation of misfolded disease proteins and other signs of cellular distress is bidirectional, and in many cases mutually exacerbating. For example, amyloid-β, a-synuclein, and mHtt all cause acute oxidative stress in neurons and/or astrocytes, and impair astroglial anti-oxidant responses. Conversely, oxidative stress promotes the aggregation of disease proteins, and contributes to age- and disease-related proteostatic collapse. Similarly, there appeara as a downward spiralling cycle of interactions between protein misfolding and neuroinflammation, which has been most extensively studied for AD. Soluble Aβ oligomers and insoluble Aβ aggregates have been shown to bind to and activate and microglia and astrocytes, stimulating a chronic low level state of neuroinflammation.