SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents
Karlstad et al., JAMA Cardiology, 2022.04
“Findings In a cohort study of 23.1 million residents across 4 Nordic countries, risk of myocarditis after the first and second doses of SARS-CoV-2 mRNA vaccines was highest in young males aged 16 to 24 years after the second dose. For young males receiving 2 doses of the same vaccine, data were compatible with between 4 and 7 excess events in 28 days per 100 000 vaccinees after second-dose BNT162b2, and between 9 and 28 per 100 000 vaccinees after second-dose mRNA-1273.
Meaning The risk of myocarditis in this large cohort study was highest in young males after the second SARS-CoV-2 vaccine dose, and this risk should be balanced against the benefits of protecting against severe COVID-19 disease.
Results Among 23 122 522 Nordic residents (81% vaccinated by study end; 50.2% female), 1077 incident myocarditis events and 1149 incident pericarditis events were identified. Within the 28-day period, for males and females 12 years or older combined who received a homologous schedule, the second dose was associated with higher risk of myocarditis, with adjusted IRRs of 1.75 (95% CI, 1.43-2.14) for BNT162b2 and 6.57 (95% CI, 4.64-9.28) for mRNA-1273. Among males 16 to 24 years of age, adjusted IRRs were 5.31 (95% CI, 3.68-7.68) for a second dose of BNT162b2 and 13.83 (95% CI, 8.08-23.68) for a second dose of mRNA-1273, and numbers of excess events were 5.55 (95% CI, 3.70-7.39) events per 100 000 vaccinees after the second dose of BNT162b2 and 18.39 (9.05-27.72) events per 100 000 vaccinees after the second dose of mRNA-1273. Estimates for pericarditis were similar.
Conclusions and Relevance Results of this large cohort study indicated that both first and second doses of mRNA vaccines were associated with increased risk of myocarditis and pericarditis. For individuals receiving 2 doses of the same vaccine, risk of myocarditis was highest among young males (aged 16-24 years) after the second dose. These findings are compatible with between 4 and 7 excess events in 28 days per 100 000 vaccinees after BNT162b2, and between 9 and 28 excess events per 100 000 vaccinees after mRNA-1273. This risk should be balanced against the benefits of protecting against severe COVID-19 disease.”
Figure 3. Myocarditis Within 28 Days After SARS-CoV-2 Vaccination in 4 Nordic Countries Among Males and Females Aged 12 Years or Older, With Pooled Estimates
Squares represent incidence rate ratios (IRRs) with 95% CIs; square size, country weight; and diamonds, pooled estimates with 95% CIs. A single vaccine name indicates first dose of that vaccine (eg, BNT162b2) and the risk of the outcome after the first dose. Vaccine names in combination indicate a vaccine schedule of first dose of the first vaccine and a second dose of the second vaccine (eg, BNT162b2, BNT162b2) and the risk of the outcome after the second dose. Model 2 adjusted for age group and sex, previous SARS-CoV-2 infection, health care worker status, nursing home resident, and comorbidity variables.
Cadegiani, ResearchGate preprint, 2022.02
Background: “Myocarditis induced by mRNA SARS-CoV-2 vaccines is a well- documented complication observed in young males, while increase of the incidence in sudden deaths among athletes have been reported and requires further investigation. Myocarditis is a major cause of sudden deaths in young male athletes. The severity and implications of these critical severe adverse effects request a thorough analysis aiming to elucidate the key mechanisms that trigger these events.”
Methods: “To demonstrate, through a review in the literature, whether there is substantiation to hypothesize that catecholamines, in a sort of ‘hypercatecholinergic state’, are the key trigger of the mRNA SARS-CoV-2 vaccines-induced myocarditis and potential sudden deaths.”
Results: “The rationale and data that support the hypothesis include: 1. mRNA COVID- 19 vaccine-induced myocarditis particularly affects young males, and, although also may also be present in COVID-19 infection, complications such as heart arrhythmia are more commonly found after vaccines; 2. Independent autopsies of deaths due to mRNA COVID-19 vaccine in different geographical regions concluded that catecholamine- triggered myocarditis was the primary cause of all cases; 3. There is a dense presence of SARS-CoV-2 mRNA and progressive production of SARS-CoV-2 spike protein in chromaffin cells in the adrenal medulla, which are the responsible for the production of catecholamines; 4. There is an overexpression of DOPA-descarboxylase enzyme, that converts dopamine into noradrenaline, when in the presence of mRNA SARS-CoV-2, leading to enhanced activity of noradrenaline; 5. Catecholamine responses are physiologically higher in youngers and in males; 6. Catecholamine responses and resting catecholamine production are higher in male athletes than in non-athletes; 7. Catecholamine response to stress and sensitivity to catecholamines are enhanced in the presence of androgens; 8. Catecholamine responses, which are already high in young male athletes, are overexpressed in vaccinated athletes, when compared to pre- vaccination levels and non-vaccinated athletes.”
Conclusion: “Epidemiological, autopsies, molecular and physiological findings unanimously and strongly suggest that a ‘hypercatecholinergic’ state is the critical predictor that triggers mRNA COVID-19 vaccine-induced myocarditis and potential increase in sudden deaths among elite athletes.”
Figure 1. Summary of the rationale and supporting data for the present hypothesis.
Gill et al., Archives of Pathology & Laboratory Medicine, 2022.02
Results: “The microscopic examination revealed features resembling a catecholamine-induced injury, not typical myocarditis pathology.”
Conclusions: “The myocardial injury seen in these post-vaccine hearts is different from typical myocarditis and has an appearance most closely resembling a catecholamine-mediated stress (toxic) cardiomyopathy. Understanding that these instances are different from typical myocarditis and that cytokine storm has a known feedback loop with catecholamines may help guide screening and therapy.”
Oster et al., JAMA, 2022.01
“Findings In this descriptive study of 1626 cases of myocarditis in a national passive reporting system, the crude reporting rates within 7 days after vaccination exceeded the expected rates across multiple age and sex strata. The rates of myocarditis cases were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.7 per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.9 per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.4 and 56.3 per million doses of the BNT162b2 vaccine and the mRNA-1273 vaccine, respectively).
Meaning Based on passive surveillance reporting in the US, the risk of myocarditis after receiving mRNA-based COVID-19 vaccines was increased across multiple age and sex strata and was highest after the second vaccination dose in adolescent males and young men.
Results Among 192 405 448 persons receiving a total of 354 100 845 mRNA-based COVID-19 vaccines during the study period, there were 1991 reports of myocarditis to VAERS and 1626 of these reports met the case definition of myocarditis. Of those with myocarditis, the median age was 21 years (IQR, 16-31 years) and the median time to symptom onset was 2 days (IQR, 1-3 days). Males comprised 82% of the myocarditis cases for whom sex was reported. The crude reporting rates for cases of myocarditis within 7 days after COVID-19 vaccination exceeded the expected rates of myocarditis across multiple age and sex strata. The rates of myocarditis were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.7 per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.9 per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.4 and 56.3 per million doses of the BNT162b2 vaccine and the mRNA-1273 vaccine, respectively). There were 826 cases of myocarditis among those younger than 30 years of age who had detailed clinical information available; of these cases, 792 of 809 (98%) had elevated troponin levels, 569 of 794 (72%) had abnormal electrocardiogram results, and 223 of 312 (72%) had abnormal cardiac magnetic resonance imaging results. Approximately 96% of persons (784/813) were hospitalized and 87% (577/661) of these had resolution of presenting symptoms by hospital discharge. The most common treatment was nonsteroidal anti-inflammatory drugs (589/676; 87%).
Conclusions and Relevance Based on passive surveillance reporting in the US, the risk of myocarditis after receiving mRNA-based COVID-19 vaccines was increased across multiple age and sex strata and was highest after the second vaccination dose in adolescent males and young men. This risk should be considered in the context of the benefits of COVID-19 vaccination.”
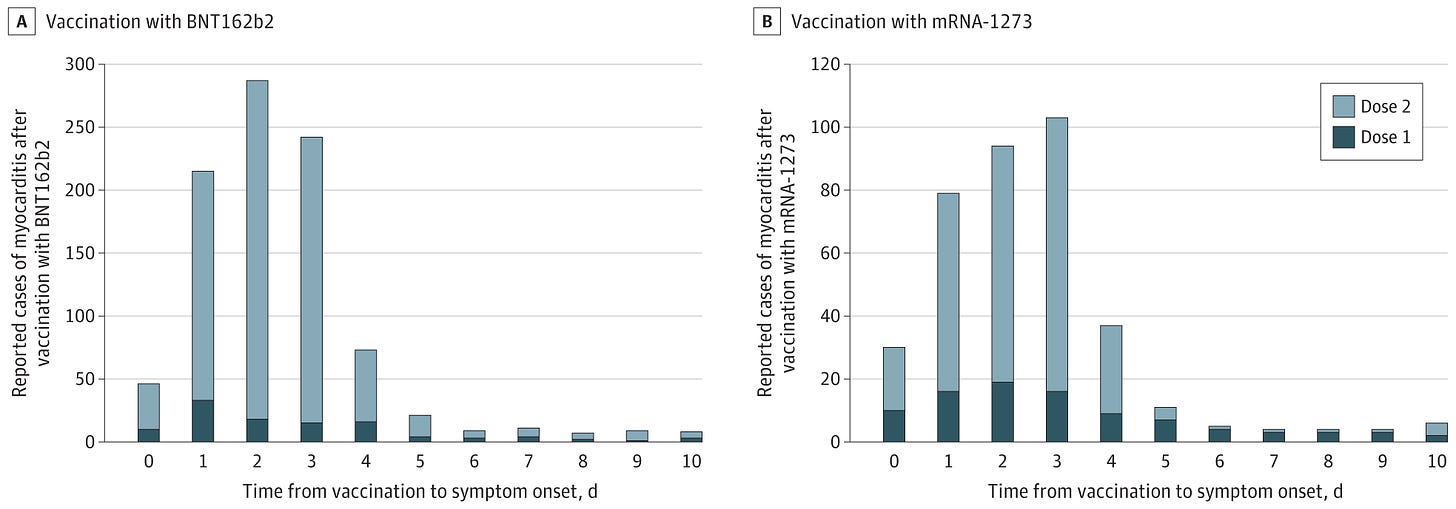
Figure 2. Cases of Myocarditis After mRNA-Based COVID-19 Vaccination by Time From Vaccination to Symptom Onset
The reports to the Vaccine Adverse Event Reporting System met the case definition of myocarditis (reported cases). Among recipients of either vaccine, there were only 13 reports or less of myocarditis beyond 10 days for any individual time from vaccination to symptom onset. The y-axis range differs between panels A and B.
A, For the BNT162b2 vaccine, there were 138 reported cases of myocarditis with known date for symptom onset and dose after 114 246 837 first vaccination doses and 888 reported cases after 95 532 396 second vaccination doses.
B, For the mRNA-1273 vaccine, there were 116 reported cases of myocarditis with known date for symptom onset and dose after 78 158 611 first vaccination doses and 311 reported cases after 66 163 001 second vaccination doses.
Avolio et al., Clinical Science, 2021.12
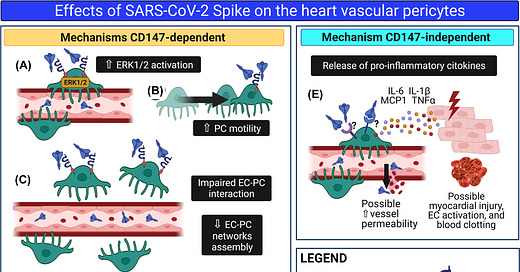
“The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a broad range of clinical responses including prominent microvascular damage. The capacity of SARS-CoV-2 to infect vascular cells is still debated. Additionally, the SARS-CoV-2 Spike (S) protein may act as a ligand to induce non-infective cellular stress. We tested this hypothesis in pericytes (PCs), which are reportedly reduced in the heart of patients with severe coronavirus disease-2019 (COVID-19). Here we newly show that the in vitro exposure of primary human cardiac PCs to the SARS-CoV-2 wildtype strain or the α and δ variants caused rare infection events. Exposure to the recombinant S protein alone elicited signalling and functional alterations, including: (1) increased migration, (2) reduced ability to support endothelial cell (EC) network formation on Matrigel, (3) secretion of pro-inflammatory molecules typically involved in the cytokine storm, and (4) production of pro-apoptotic factors causing EC death. Next, adopting a blocking strategy against the S protein receptors angiotensin-converting enzyme 2 (ACE2) and CD147, we discovered that the S protein stimulates the phosphorylation/activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) through the CD147 receptor, but not ACE2, in PCs. The neutralisation of CD147, either using a blocking antibody or mRNA silencing, reduced ERK1/2 activation, and rescued PC function in the presence of the S protein. Immunoreactive S protein was detected in the peripheral blood of infected patients. In conclusion, our findings suggest that the S protein may prompt PC dysfunction, potentially contributing to microvascular injury. This mechanism may have clinical and therapeutic implications.”
Figure 11 The SARS-CoV-2 S protein alters cardiac PC function
“Schematic summary of the research. We hypothesise that in patients with acute COVID-19, S protein molecules are cleaved from the virus particle and released from the respiratory system into the bloodstream. Through the circulation, isolated S protein reaches all organs of the body, including the heart. Here, the interaction of the S protein with the CD147 receptor on cardiac PCs triggers the ERK1/2 signalling (A) and provokes PC dysfunction, including increased cell motility (B) and decreased cooperation with coronary ECs during angiogenesis. (C). In addition, the S protein–CD147 interaction prompts cardiac PCs to release pro-apoptotic factors, which cause EC death (D). Finally, through a mechanism CD147-independent, the S protein induces PCs to release pro-inflammatory cytokines, which include MCP1, IL-6, IL-1β, and TNFα (E). These cytokines can damage neighbouring cardiomyocytes and activate ECs, potentially triggering blood clotting and increasing vascular permeability.”
Patone et al., Nature Medicine, 2021.12
“We found increased risks of myocarditis associated with the first dose of ChAdOx1 and BNT162b2 vaccines and the first and second doses of the mRNA-1273 vaccine over the 1–28 days postvaccination period, and after a SARS-CoV-2 positive test. We estimated an extra two (95% confidence interval (CI) 0, 3), one (95% CI 0, 2) and six (95% CI 2, 8) myocarditis events per 1 million people vaccinated with ChAdOx1, BNT162b2 and mRNA-1273, respectively, in the 28 days following a first dose and an extra ten (95% CI 7, 11) myocarditis events per 1 million vaccinated in the 28 days after a second dose of mRNA-1273. This compares with an extra 40 (95% CI 38, 41) myocarditis events per 1 million patients in the 28 days following a SARS-CoV-2 positive test. We also observed increased risks of pericarditis and cardiac arrhythmias following a positive SARS-CoV-2 test. Similar associations were not observed with any of the COVID-19 vaccines, apart from an increased risk of arrhythmia following a second dose of mRNA-1273. Subgroup analyses by age showed the increased risk of myocarditis associated with the two mRNA vaccines was present only in those younger than 40.”
Fig. 2: Number of excess events due to exposure per 1 million exposed
When IRR did not show a significant increase of incidence over the 1–28 days postvaccination or a SARS-CoV-2 positive test, absolute measures are not given.
Rose and McCullough, Current Problems in Cardiology, 2021.11 (Withdrawn)
“Following the global rollout and administration of the Pfizer Inc./BioNTech BNT162b2 and Moderna mRNA-1273 vaccines on December 17, 2020, in the United States, and of the Janssen Ad26.COV2.S product on April 1st, 2021, in an unprecedented manner, hundreds of thousands of individuals have reported adverse events (AEs) using the Vaccine Adverse Events Reports System (VAERS). We used VAERS data to examine cardiac AEs, primarily myocarditis, reported following injection of the first or second dose of the COVID-19 injectable products. Myocarditis rates reported in VAERS were significantly higher in youths between the ages of 13 to 23 (p<0.0001) with ∼80% occurring in males. Within 8 weeks of the public offering of COVID-19 products to the 12-15-year-old age group, we found 19 times the expected number of myocarditis cases in the vaccination volunteers over background myocarditis rates for this age group. In addition, a 5-fold increase in myocarditis rate was observed subsequent to dose 2 as opposed to dose 1 in 15-year-old males. A total of 67% of all cases occurred with BNT162b2. Of the total myocarditis AE reports, 6 individuals died (1.1%) and of these, 2 were under 20 years of age - 1 was 13. These findings suggest a markedly higher risk for myocarditis subsequent to COVID-19 injectable product use than for other known vaccines, and this is well above known background rates for myocarditis. COVID-19 injectable products are novel and have a genetic, pathogenic mechanism of action causing uncontrolled expression of SARS-CoV-2 spike protein within human cells. When you combine this fact with the temporal relationship of AE occurrence and reporting, biological plausibility of cause and effect, and the fact that these data are internally and externally consistent with emerging sources of clinical data, it supports a conclusion that the COVID-19 biological products are deterministic for the myocarditis cases observed after injection.”
Dr. McCullough sues Elsevier for retracting:
A Report on Myocarditis Adverse Events in the U.S. Vaccine Adverse Events Reporting System (VAERS) in Association with COVID-19 Injectable Biological Products
Rose & McCullough, Current Problems in Cardiology, 2021.11
“VAERS, Myocarditis, COVID Vaccines & so much more”, Dr. Jessica Rose, 2021.12, 📹
“Hearts on fire fueled by mRNA”, Dr. Peter McCullough,🎙
Chua et al., Clinical Infectious Diseases, 2021.11
“Results Between 14 June 2021 and 4 September 2021, 33 Chinese adolescents who developed acute myocarditis/pericarditis following Comirnaty vaccination were identified. In total, 29 (87.88%) were male and 4 (12.12%) were female, with a median age of 15.25 years. And 27 (81.82%) and 6 (18.18%) cases developed acute myocarditis/pericarditis after receiving the second and first dose, respectively. All cases are mild and required only conservative management. The overall incidence of acute myocarditis/pericarditis was 18.52 (95% confidence interval [CI], 11.67–29.01) per 100 000 persons vaccinated. The incidence after the first and second doses were 3.37 (95% CI, 1.12–9.51) and 21.22 (95% CI, 13.78–32.28 per 100 000 persons vaccinated, respectively. Among male adolescents, the incidence after the first and second doses were 5.57 (95% CI, 2.38–12.53) and 37.32 (95% CI, 26.98–51.25) per 100 000 persons vaccinated.
Conclusions There is a significant increase in the risk of acute myocarditis/pericarditis following Comirnaty vaccination among Chinese male adolescents, especially after the second dose.”
Høeg et al., medRxiv, 2021.09
Results: “A total of 257 CAEs were identified. Rates per million following dose 2 among males were 162.2 (ages 12-15) and 94.0 (ages 16-17); among females, rates were 13.0 and 13.4 per million, respectively. For boys 12-15 without medical comorbidities receiving their second mRNA vaccination dose, the rate of CAE is 3.7 to 6.1 times higher than their 120-day COVID- 19 hospitalization risk as of August 21, 2021 (7-day hospitalizations 1.5/100k population) and 2.6-4.3-fold higher at times of high weekly hospitalization risk (7-day hospitalizations 2.1/100k), such as during January 2021. For boys 16-17 without medical comorbidities, the rate of CAE is currently 2.1 to 3.5 times higher than their 120-day COVID-19 hospitalization risk, and 1.5 to 2.5 times higher at times of high weekly COVID-19 hospitalization.”
Figure 7. Harm-benefit analysis for second dose of mRNA vaccine cardiac adverse event (CAE) in boys by age vs. COVID-19 hospitalization risk in the context of disease prevalence and presence of ≥ 1 comorbidity.